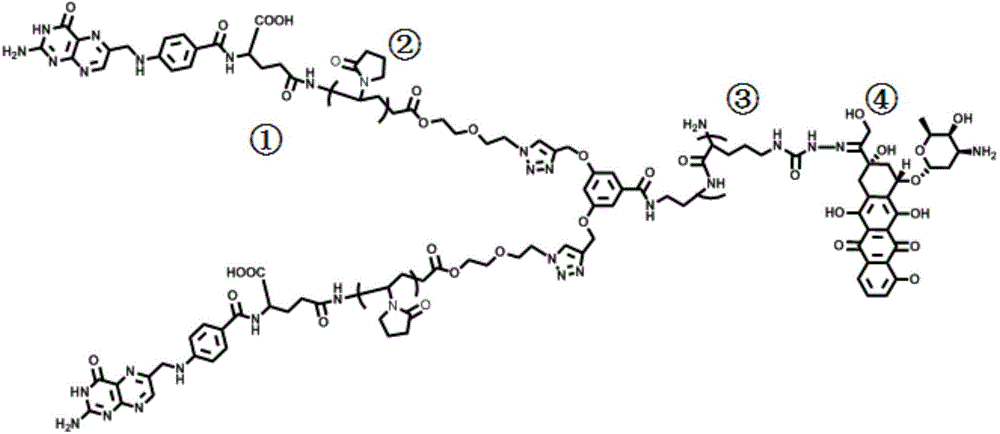
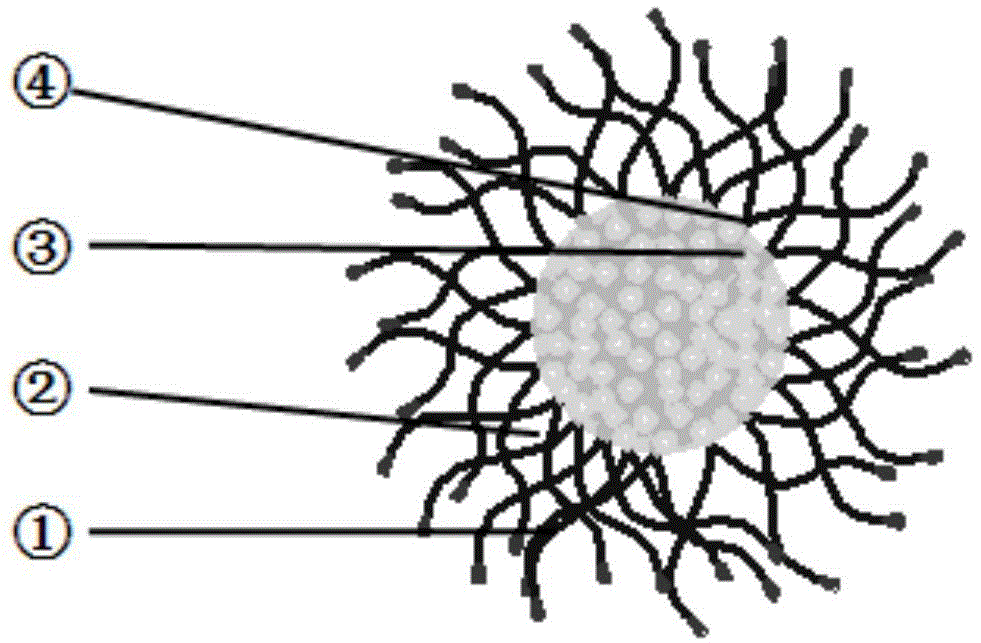
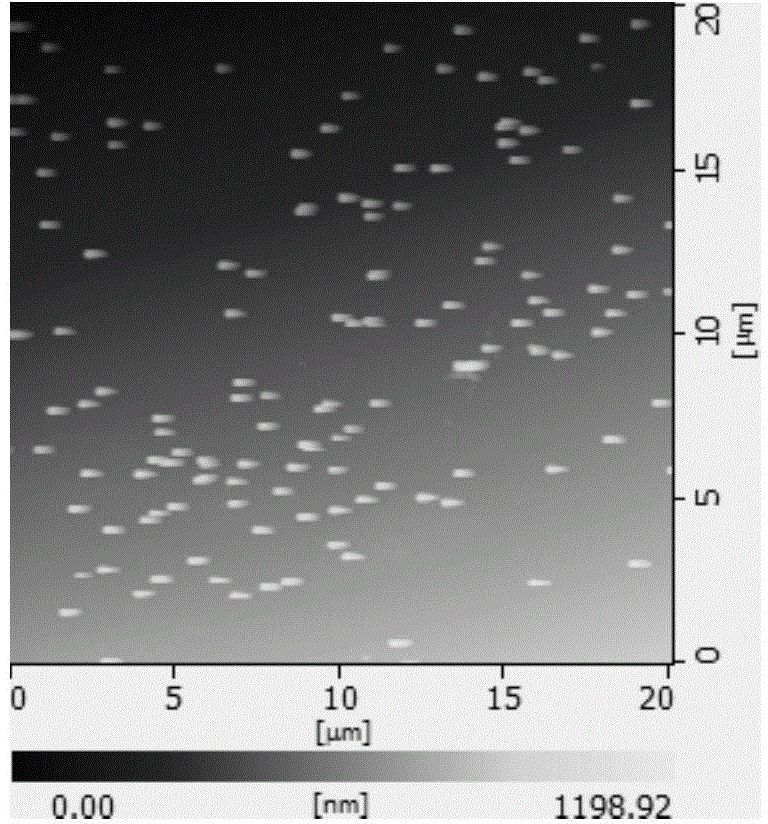
Novel anti-cancer polymer drug
A technology of polymers and polymer materials, applied in the direction of nano-medicines, anti-tumor drugs, drug combinations, etc., can solve the problems of reduced water solubility of drug molecules, high synthesis costs, poor biocompatibility, etc., to reduce toxic side effects, Good biocompatibility and large loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] ① Preparation of azide small molecule initiator
[0045] Step 1: Dissolve 5mL 2-chloroethoxyethanol in 25mL methyl ethyl ketone, add 4.5g NaN to the solution 3 , 2.5gBu 4 NI, 10 mg of dicyclohexane-18-crown-6, heat the mixture to boiling and stir for 24 hours. The mixture was filtered and the precipitate was thoroughly rinsed with acetone. The resulting mixed solution is the crude product. After concentrating the mixed solution, o C is distilled to obtain a pure product. The resulting 2-azidoethoxyethanol 1 H NMR (CDCl 3 ): 3.70 (t, 2 H, C H 2 OH), 3.65 (t, 2 H, HOCH 2 C H 2 O), 3.56 (t, 2H, N 3 CH 2 C H 2 O), 3.37 (t, 2H, C H 2 N 3 ), and 2.56 (s, 1H, OH).
[0046] Step 2: Dissolve 2 g of 2-azidoethoxyethanol and 5.09 g of α-chloro acid chloride prepared in step 1 in 30 mL of dichloromethane, transfer the reaction system to the ice field, and transfer 6.8 g of dicyclohexyl The carbodiimide was slowly added to the reaction vessel and stirred. 0.4 g of dimethylaminopyrid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com