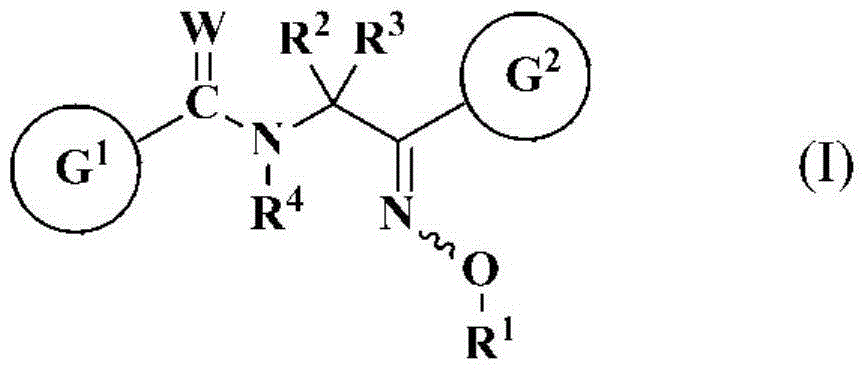
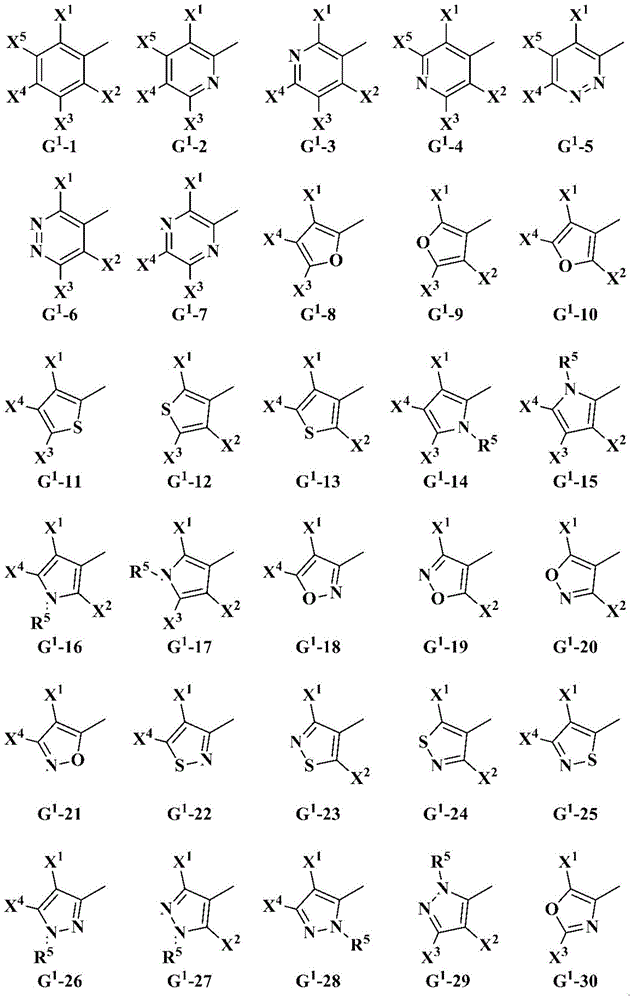
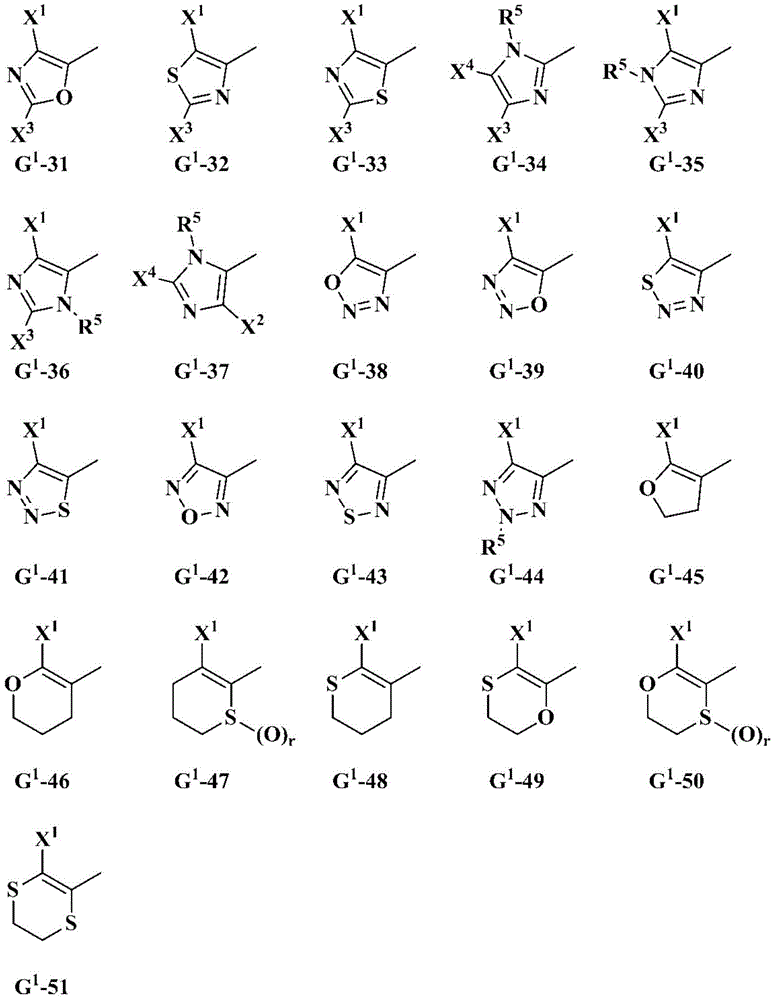
Oxime-substituted amide compound and pest control agent
A technology of oxime-substituted amides and compounds, applied in the field of pest control agents, can solve problems such as changing the lifespan of eukaryotes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0926] Hereinafter, the present invention will be further described in detail by specifically describing the synthesis examples and test examples of the compounds of the present invention as examples, but the present invention is not limited thereto.
Synthetic example 1
[0929] (Z)-N-[2-(2,4-dichlorophenyl)-2-(methoxyimino)ethyl]-2-(trifluoromethyl)benzamide (compound No. 1-004).
[0930] Step 1; Production of 2-bromo-1-(2,4-dichlorophenyl)ethanone-O-methyloxime
[0931] To a solution of 4.00 g of 2-bromo-1-(2,4-dichlorophenyl)ethanone in 20 ml of ethanol was added 1.25 g of methoxyamine hydrochloride, followed by stirring at room temperature for 12 hours. After the reaction, the solvent was distilled off under reduced pressure, 20 ml of water was added to the residue, and extraction was performed with ethyl acetate (20 ml×2). The organic layers were combined, washed with water (20ml×1), dehydrated and dried in the order of saturated brine, followed by anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain the crude target compound 3.85 in the form of a light yellow oily substance. g. It was used directly in the next procedure without further purification.
[0932] 1 H NMR (CDCl 3 , Me 4 Si, 300MHz)...
Synthetic example 2
[0945] (Z)-N-[2-(3,5-dichloropyridin-2-yl)-2-(ethoxyimino)ethyl]-2-(trifluoromethyl)benzamide (the present invention Compound No. 2-120).
[0946] Step 1; Production of 1-(3,5-dichloropyridin-2-yl)ethanone
[0947] To a solution of 20 g of 3,5-dichloropyridine-2-carbonitrile in 150 ml of tetrahydrofuran, 139 ml of a 1 M tetrahydrofuran solution of methylmagnesium bromide was added dropwise with stirring under ice cooling, and stirred at this temperature for 1 hour. After the reaction, 15ml of concentrated hydrochloric acid and 100ml of water were added to the reaction mixture, extracted with ethyl acetate (100ml×2), the organic layers were combined, washed with water (100ml×1), followed by saturated saline, followed by anhydrous sulfuric acid Dehydration and drying were performed sequentially with sodium, and the solvent was distilled off under reduced pressure. The residue was dissolved in 40 ml of ethyl acetate and 10 ml of hexane, 20 g of silica gel was added, stirred at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com