Preparation method of recombinant feruloyl esterase
A technology of ferulic acid esterase and recombinant plasmid, which is applied in the field of preparation of recombinant ferulic acid esterase, can solve the problem of difficult to meet the requirements of industrial production, complicated separation and extraction process of ferulic acid esterase, ferulic acid esterase enzyme activity and low ferulic acid esterase. The expression amount does not meet the needs of the industry and other problems, so as to achieve the effect of improving the expression efficiency and expression amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of recombinant ferulic acid esterase
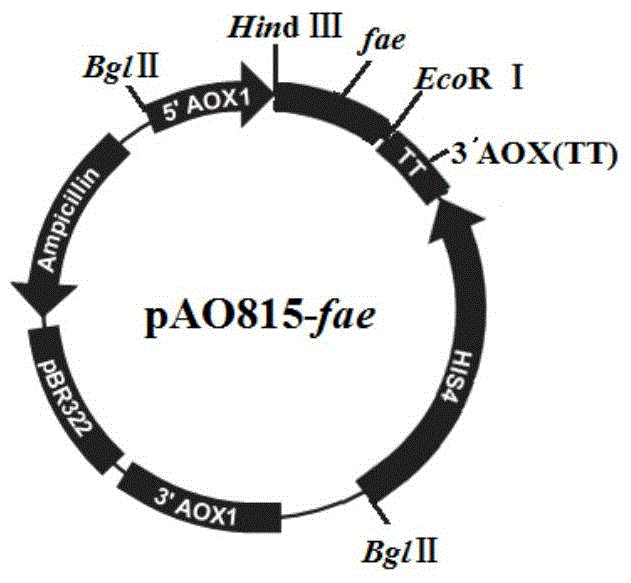
[0032] 1) Obtaining the target gene fae: screening the amino acid sequence of ferulic acid esterase O42807 with high enzymatic activity in the UniProt database, which was isolated from Aspergillus niger (Aspergillus niger) CBS120.49 by De Vries RP et al. It belongs to extracellular enzyme; the amino acid sequence of ferulic acid esterase O42807 is shown in SEQ ID NO.2, according to the mature peptide sequence of O42087 (as shown in SEQ ID NO.2, 22-281 is the mature peptide sequence) The gene sequence of ferulic acid esterase was designed by standard codon optimization of Pichia pastoris, and HindⅢ and EcoRI enzyme cleavage sites were respectively introduced into the two ends of the gene sequence, and the gene sequence shown in SEQ ID NO.1 was chemically synthesized and named as Fae;
[0033] 2) Construct the pAO815-fae expression vector: use HindⅢ and EcoRI to double-digest the synthetic gene sequence fae...
Embodiment 2
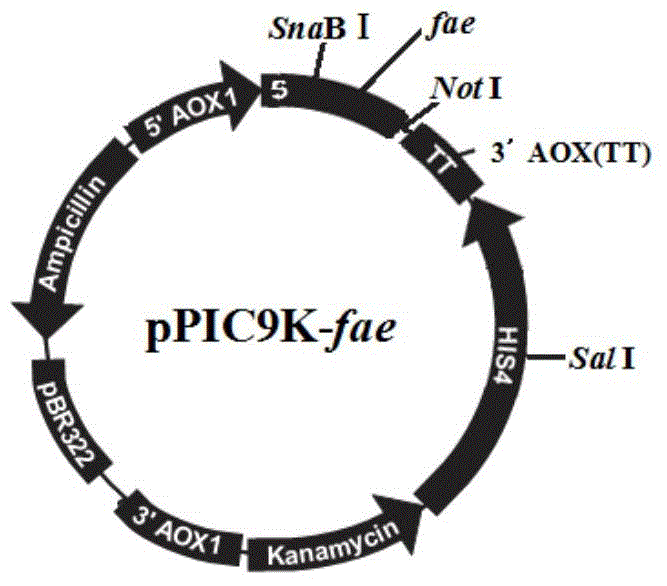
[0036] Example 2: Identification of GS115 / pPIC9K-fae
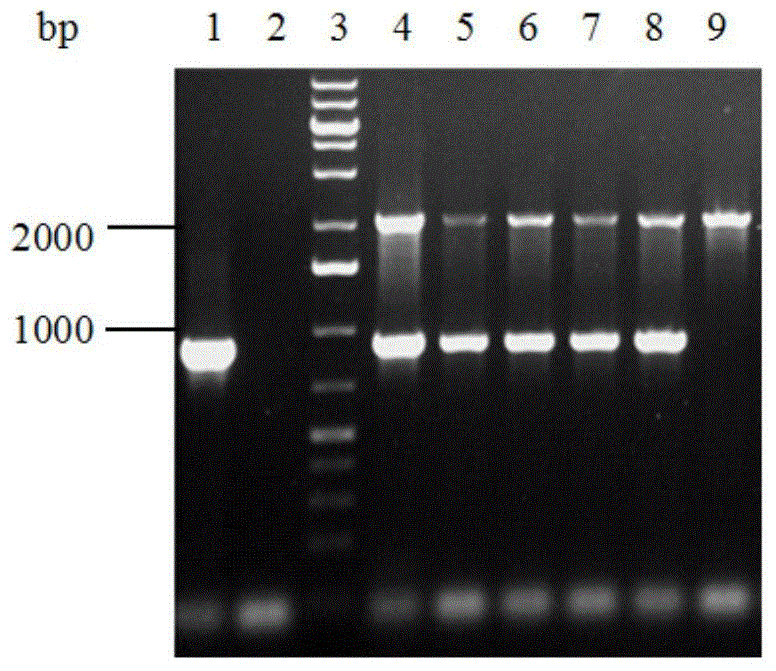
[0037] Extract the genomic DNA of the GS115 / pPIC9K-fae obtained in Example 1, utilize universal primer 5'AOX and 3'AOX to carry out PCR identification, the result is as follows image 3 As shown, GS115 / pPIC9K-fae was constructed successfully.
Embodiment 3
[0038] Example 3: Determination of the expression level of GS115 / pPIC9K-fae
[0039] For the induced expression of recombinant yeast GS115 / pPIC9K-fae, refer to Invitrogen’s Pichia pastoris operation and expression manual. Every 12h or 24h of methanol induction, the fermentation supernatant was taken for SDS-PAGE analysis to determine the expression of recombinant ferulic acid esterase. Such as Figure 4 As shown, a clear band was obtained at 42kDa, and there were almost no mixed bands, preliminarily indicating that fae was successfully expressed. Utilize Bradford method to measure recombinant ferulic acid esterase protein content, HPLC method measures enzyme activity, and assay result is as follows Figure 5 As shown, the enzyme activity is 4.7U / mL, and the specific enzyme activity is 31.4U / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com