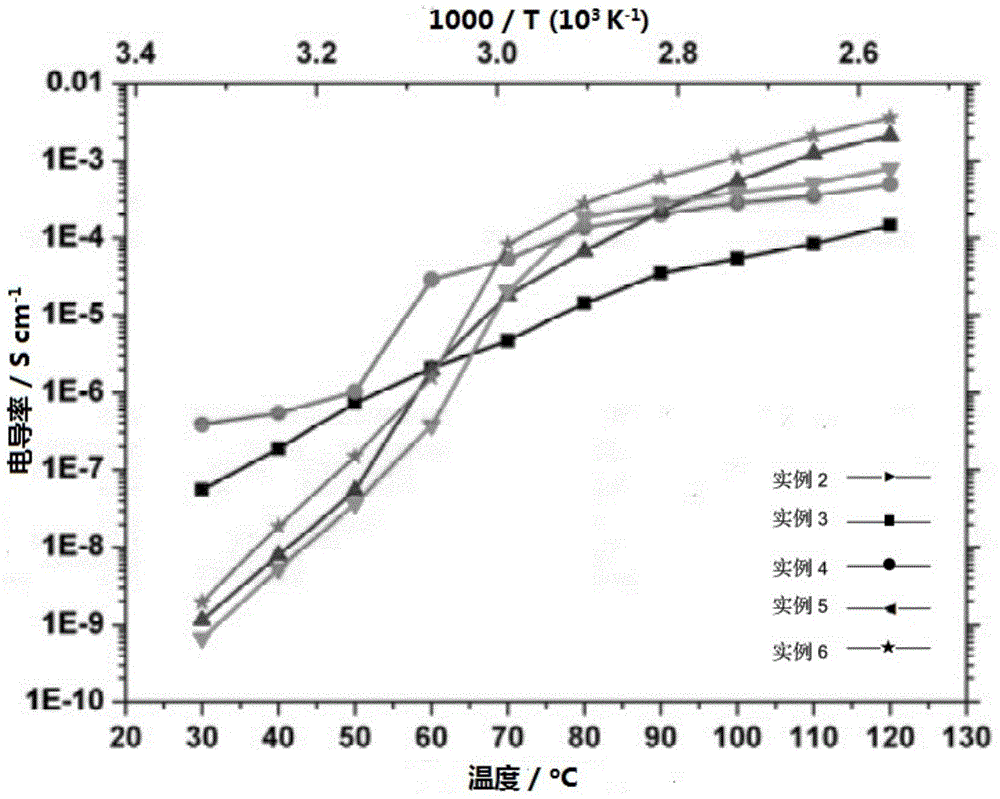
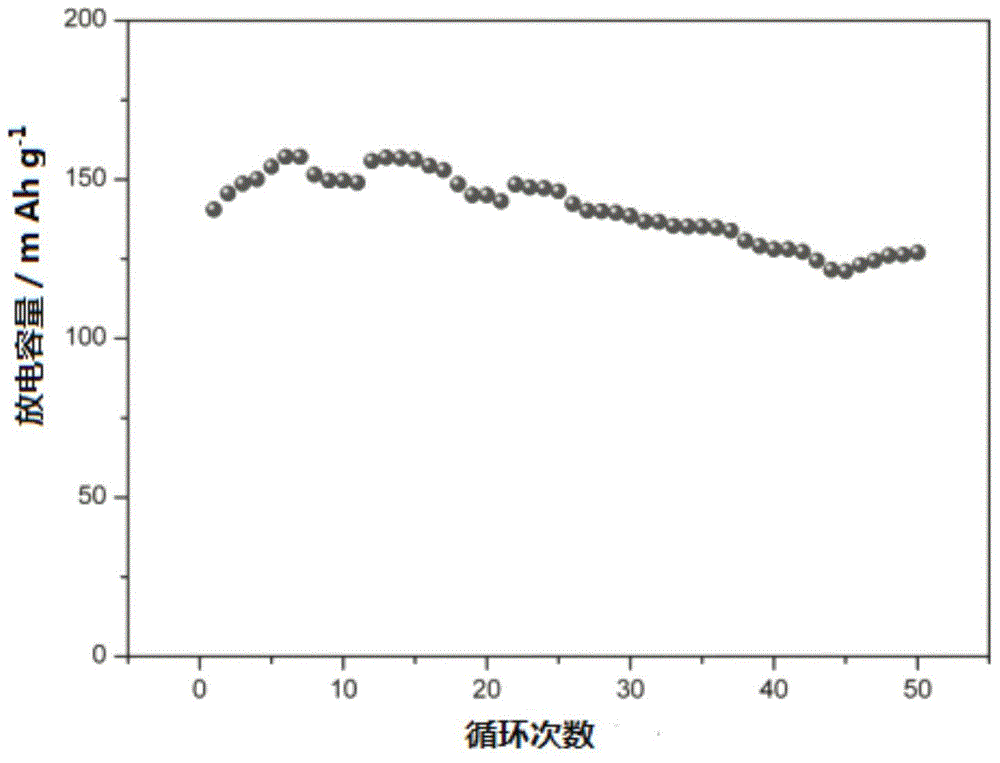
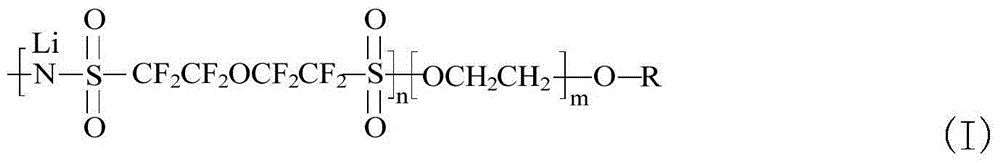Novel solid-state electrolyte membrane material capable of being used as lithium ion battery as well as preparation method and application thereof
A solid-state electrolyte membrane and lithium-ion battery technology, applied in the field of polymer materials, can solve the problems of high battery polarization, reduced capacity and battery life, etc., and achieve the effects of good mechanical strength, improved migration number, and high electrochemical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step 1: In an inert atmosphere, the fluorine-containing monomer ClSO 2 CF 2 CF 2 OCF 2 CF 2 SO 2 NH 2 (8g, 20mmol) was dissolved in acetonitrile (100mL), to this solution was added Cs 2 CO 3 (26g, 80mmol), polyethylene oxide monomethyl ether (M n =400, 8g, 20mmol) system was heated to 40°C and stirred for 4d, the reaction solution was filtered, and the filtrate was concentrated to obtain block copolymer PFSICs-b-PEO (18g) in the form of cesium salt.
[0037] Step 2: Dissolve the block copolymer PFSICs-b-PEO (18 g) obtained in step 1 in the form of cesium salt in acetonitrile (16 mL), and dissolve LiBF 4 (1.9g, 20mmol) was dissolved in acetonitrile (2mL), added dropwise to the acetonitrile solution containing the polymer, placed at 0°C and stirred for 24h, filtered to obtain the block copolymer PFSILi-b-PEO in the form of lithium salt, Its molecular structural formula is shown in formula (III).
[0038]
Embodiment 2
[0040] Step 1: In an inert atmosphere, the fluorine-containing monomer ClSO 2 CF 2 CF 2 OCF 2 CF 2 SO 2 NH 2 (4g, 10mmol) was dissolved in acetonitrile (80mL), and CsF (15g, 100mmol) and polyethylene oxide (M n =10000, 10g, 1mmol), the system was heated to 60°C and stirred for 10d, after which the reaction liquid was filtered, and the filtrate was concentrated to obtain block copolymer PFSICs-b-PEO (15g) in the form of cesium salt.
[0041] Step 2: Dissolve the block copolymer PFSICs-b-PEO (15 g) obtained in step 1 in the form of cesium salt in dichloromethane (140 mL), and dissolve LiClO 4 (1.05g, 10mmol) was dissolved in dichloromethane (10mL), added dropwise to the dichloromethane solution containing the polymer, stirred at 20°C for 1h, and filtered to obtain the block copolymer PFSILi- b-PEO, its molecular structure is shown in formula (IV).
[0042]
Embodiment 3
[0044] Step 1: In an inert atmosphere, the fluorine-containing monomer ClSO 2 CF 2 CF 2 OCF 2 CF 2 SO 2 NH 2 (8g, 20mmol)﹑polyethylene oxide (M n =200, 4g, 20mmol) and KF (2.3g, 40mmol) were dissolved in acetonitrile (40mL), the solution was heated to 60°C and stirred for 10d, the reaction solution was filtered, and the filtrate was concentrated to obtain a block copolymer in the form of potassium salt PFSIK-b-PEO-b-PFSIK (10g).
[0045] Step 2: Dissolve the block copolymer PFSIK-b-PEO (10 g) obtained in step 1 in the form of potassium salt in chloroform (8 mL), and dissolve LiNO 3 (1.4g, 20mmol) was dissolved in chloroform (2mL), added dropwise to the chloroform solution containing the polymer, stirred at 50°C for 20h, and filtered to obtain the block copolymer PFSILi-b-PEO- b-PFSILi, its molecular structure formula is shown in formula (V).
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com