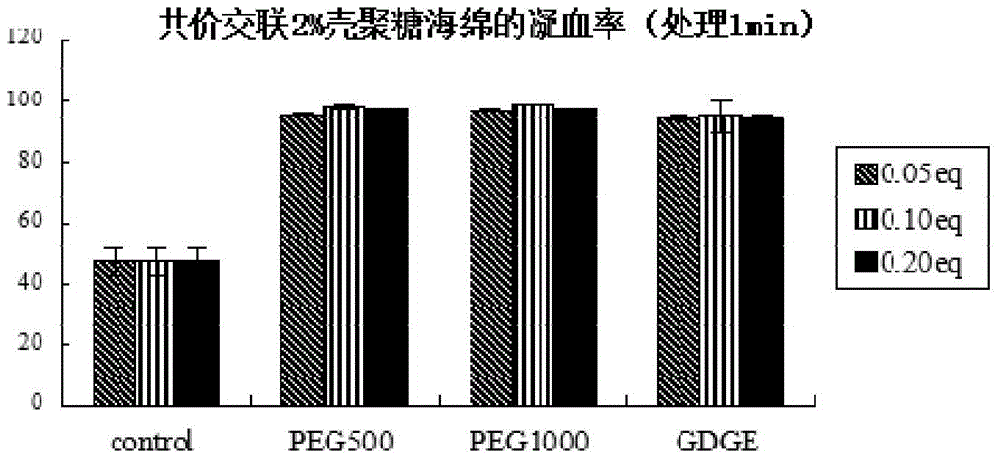
Chitosan hemostatic material formed through covalent crosslinking and preparation method thereof
A technology of covalent cross-linking and hemostatic materials, which is applied in the fields of medical science, absorbent pads, bandages, etc., can solve the problems such as the inability to fully meet the clinical needs, and achieves low production cost, safe cross-linking agent, and simple and easy preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the chitosan hemostatic material formed by covalent crosslinking, the steps are as follows:
[0034] 1) Chitosan is made into an aqueous solution;
[0035] 2) Add the cross-linking agent into the chitosan aqueous solution, stir and mix thoroughly, then pour into the mold and freeze-dry;
[0036] The cross-linking agent is polyethylene glycol diglycidyl ether.
[0037] The chemical formula of described polyethylene glycol diglycidyl ether is:
[0038] .
[0039] Preferably, n=1-20, more preferably, n=1 or 10 or 20.
[0040] The molar ratio of polyethylene glycol diglycidyl ether to monosaccharide units in chitosan molecules is 0.001:1-1:1; preferably, it is 0.05:1 or 0.10:1 or 0.20:1. The determination of the molar amount of monosaccharide units in a chitosan molecule is common knowledge.
[0041] The chitosan solution is an aqueous solution of chitosan formulated with an aqueous solution, an aqueous solution containing an organic solvent...
Embodiment 1
[0048] Weigh chitosan (viscosity 100mPa s, degree of deacetylation 90%) (0.34g, molar weight of monosaccharide unit is 21.2mmol) dissolved in 2% acetic acid solution (v / v, 17ml) to obtain 2% chitosan Sugar solution (w / v), shaking at 40°C for 24 hours to dissolve completely. Add polyethylene glycol diglycidyl ether (n = 1) (18.4mg, 0.05eq), mechanically stir and mix well, pour into a mold with a diameter of 6cm and let it stand for 12h, freeze-dry to obtain a sponge-like product with a thickness of 5mm .
Embodiment 2
[0050] The procedure is exactly the same as in Example 1, except that the amount of polyethylene glycol diglycidyl ether (n=1) added is 36.8mg (0.10eq).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com