Iseganan quick-release film agent
A technology of esgenan and film formulations, applied in the field of immediate release film formulations, to achieve the effects of good taste, strong tolerance, and not easy to be decomposed by protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
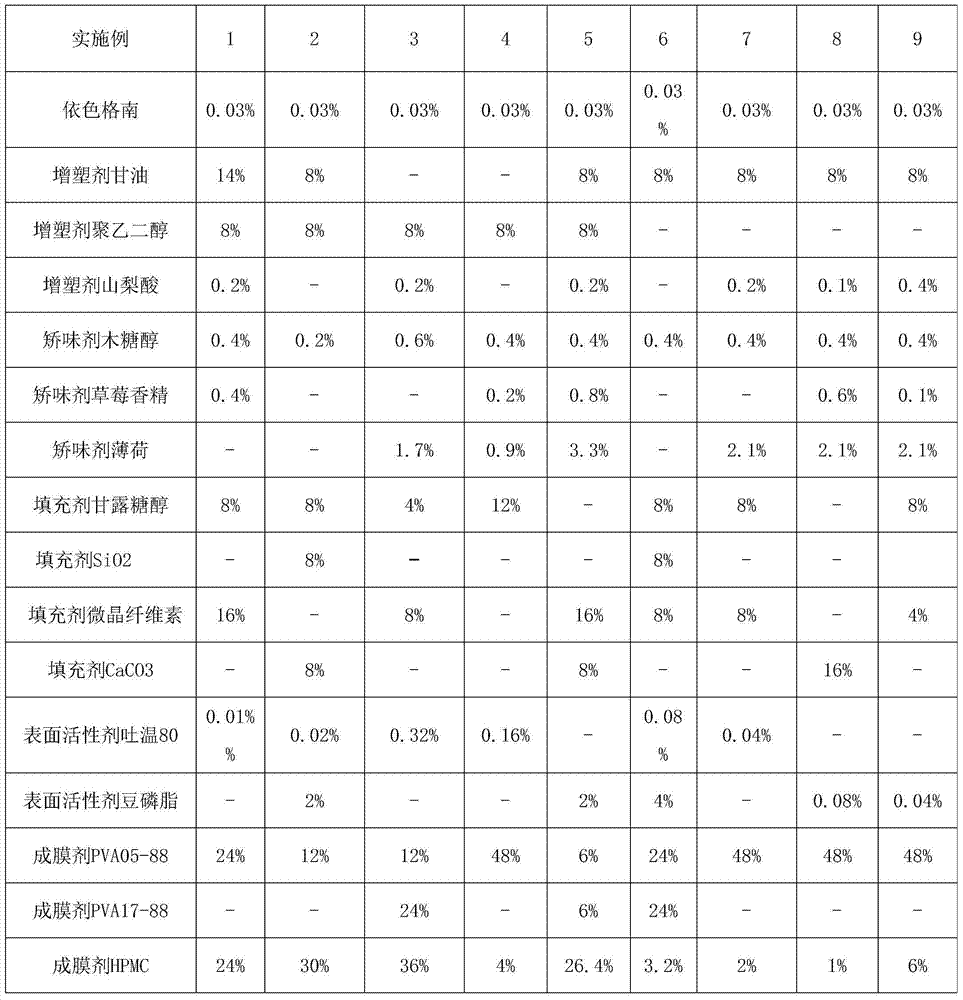
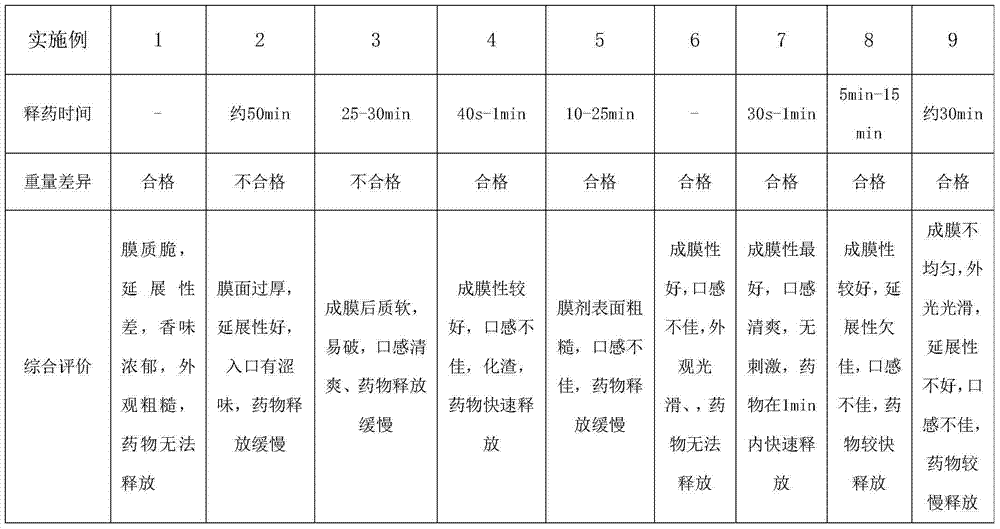
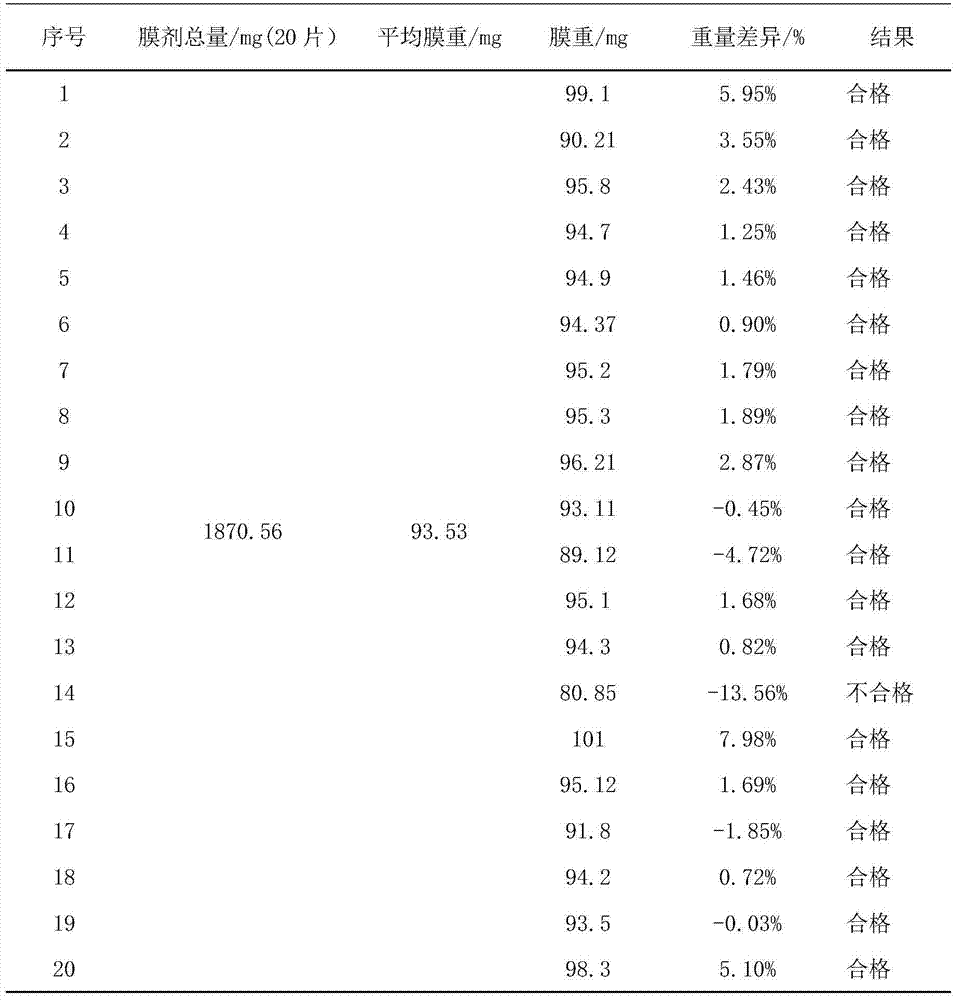
[0027] Embodiment 1 The preparation of quick-release film of the present invention (each embodiment prescription is as the criterion with Table 1)
[0028] Immediate Release Film Prescription
[0029] Estherglam 0.03% Glycerin 14% Polyethylene Glycol 8% Sorbic Acid 0.2%
[0030] Xylitol 0.4%, Strawberry Flavor 0.4%, Mannitol 8%, Microcrystalline Cellulose 16%
[0031] Tween 80 0.01% PVA05-88 24% HPMC 24%
[0032] The balance is phosphate buffer (PH6.8)
[0033] Preparation method:
[0034] 1. Soak PVA05-88 in enough buffer solution to make it fully swell, heat the fully swollen PVA05-88 at 65°C-80°C for 2 hours to dissolve, and filter;
[0035] 2. Mixing: Slowly add other excipients except the flavoring agent into the dissolved PVA05-88, stir while adding, then add the flavoring agent and stir slowly until the solution cools to 40-44°C, then gradually add the Segnam, stirring while adding;
[0036] 3. Add buffer solution to make a sufficient amount, and stir well to make...
Embodiment 2
[0037] Embodiment 2 The preparation of quick-release film agent of the present invention
[0038] Immediate Release Film Prescription
[0039] Estherglam 0.03% Glycerin 8% Macrogol 8% Xylitol 0.2%
[0040] Mannitol 8% Silicon Dioxide 8% Calcium Carbonate 8% Tween 80 0.02%
[0041] Soy Lecithin 2% PVA05-88 12% HPMC 30%
[0042] The balance is phosphate buffer (PH6.8)
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0044] Embodiment 3 The preparation of quick-release film agent of the present invention
[0045] Immediate Release Film Prescription
[0046] Estherglam 0.03% Macrogol 8% Sorbic Acid 0.2%
[0047] Xylitol 0.6% Peppermint 1.7% Mannitol 4% Microcrystalline Cellulose 8%
[0048] Tween 80 0.32% PVA05-88 12% PVA17-88 24%
[0049] HPMC 36% balance is phosphate buffer (PH6.8)
[0050] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com