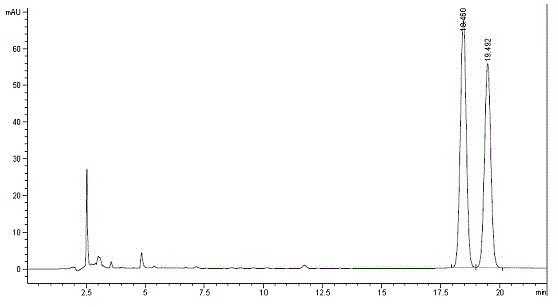
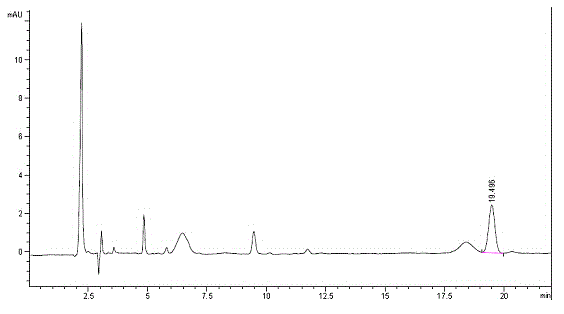
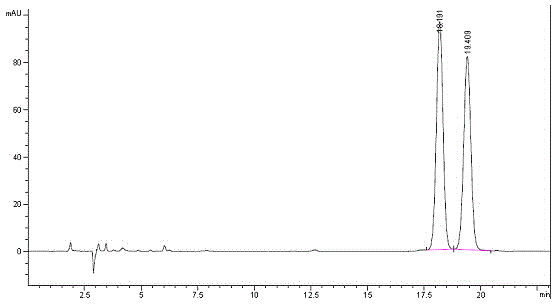
Method for performing pre-column derivation high performance liquid chromatography chiral resolution on DL-menthol by using chiral derivation reagent
A technology of high performance liquid chromatography and derivatization reagent, which is applied in the field of pre-column derivatization high performance liquid chromatography analysis and separation, can solve the problems of high cost, difficult recovery, low sensitivity, etc., and achieves low cost, accurate separation and separation, and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The synthetic method of D-mandelic acid-DL-menthyl ester used in each embodiment of the present invention, (+)-naproxen-DL-menthyl ester, concrete steps are as follows:
[0034] Add chiral aromatic acid, DL-menthol and p-toluenesulfonic acid monohydrate sequentially into a 250 mL three-neck flask equipped with a thermometer, water separator, spherical condenser and drying tube, and the molar ratio is 1.2:1:0.05 , dissolved in 100 mL toluene, add toluene to the water separator, heat to 110 o C was stirred and refluxed overnight, and TLC detected that the reaction of the raw materials was complete. Cool the reaction system to room temperature, add 20 mL of water to the system to quench the reaction, stir for 10 min and then separate the liquids, extract the aqueous phase with ethyl acetate, and wash the combined organic phase with saturated sodium bicarbonate solution and saturated brine in sequence , dried over anhydrous magnesium sulfate and concentrated to obtain crud...
Embodiment 1
[0037] A method for performing pre-column derivatization high performance liquid chromatography chiral resolution of DL-menthol by using a chiral derivatization reagent, the specific steps are as follows:
[0038] (1) Add chiral aromatic acid D-mandelic acid (5.84 g, 38.39 mmol), DL-menthol (5 g, 31.99 mmol) and catalyst p-toluenesulfonic acid monohydrate (0.31 g, 1.60 mmol), calculated by molar ratio, that is, chiral aromatic acid: DL-menthol: catalyst monohydrate p-toluenesulfonic acid is 1.2:1:0.05, then add 100 mL of toluene as solvent, shake well and add magneton, install Water separator, spherical condenser and drying pipe, fill the water separator with toluene, heat it at about 110°C, stir and reflux, condense and separate the water. After the reaction is completed, the water in the water separator will no longer increase. Cool the reaction system to room temperature, add 20 mL of water, separate the organic layer, wash the organic layer with saturated sodium bicarbonat...
Embodiment 2
[0057] A method for performing pre-column derivatization high performance liquid chromatography chiral resolution of DL-menthol by using a chiral derivatization reagent, the specific steps are as follows:
[0058]In a 250mL three-necked flask, the chiral aromatic acid (+)-naproxen (8.84 g, 38.39 mmol), DL-menthol (5 g, 31.99 mmol) and the catalyst p-toluenesulfonic acid monohydrate (0.31 g, 1.60 mmol), calculated by molar ratio, that is, chiral aromatic acid: DL-menthol: catalyst monohydrate p-toluenesulfonic acid is 1.2:1:0.05, then add 100mL of toluene as solvent, shake well and add magnets, install Water tank, spherical condenser and drying tube, fill the water separator with toluene, heat it at about 110°C, stir and reflux, condense and separate the water. After the reaction is completed, the water in the water separator will no longer increase. Cool the system to room temperature, add 20mL of water, separate the organic layer, wash the organic layer with saturated sodium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com