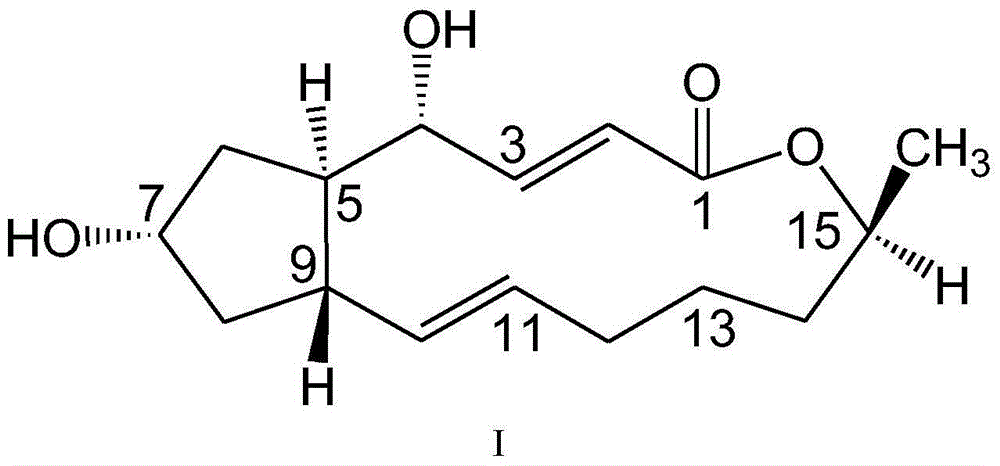
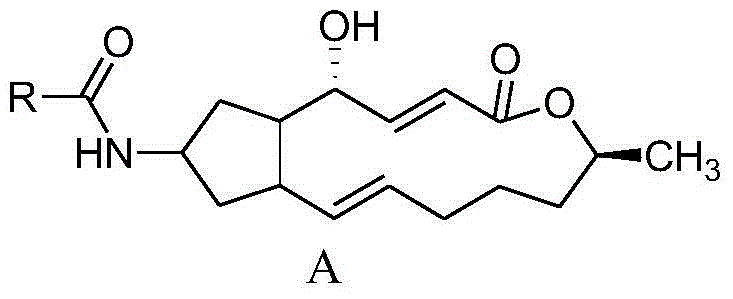
7-amide-brefeldin a derivative as well as preparation method and application of 7-amide-brefeldin a derivative
A technology of brefeldin and fideloidin, which is applied in the fields of drug combination, tripeptide composition, organic chemistry, etc., can solve the problems of short plasma half-life, inability to apply anti-tumor therapeutic reagents, and unsatisfactory problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of 7-O-methylsulfonyl-BFA (formula VI)
[0064]
[0065] Under nitrogen protection, 6 mL of pyridine was added to a 50 mL round-bottomed flask with a magnetic stirrer, then BFA (500 mg, 1.79 mmol), and then triethylamine (240 mg, 2.38 mmol) was added to start stirring at -20°C; Methanesulfonyl chloride (273mg, 2.38mmol) was added dropwise to the mixture (dropwise for 10min), and after the dropwise reaction was completed, it was reacted at -20°C for 2h to terminate the reaction. After the reaction solution was concentrated, 30ml of ethyl acetate was added for dilution, washed with 5% citric acid solution (2×10ml), saturated sodium bicarbonate solution (2×10ml), saturated sodium chloride solution (2×10ml), and organic phase, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition that the developer ethyl acetate (E):petr...
Embodiment 2
[0068] Example 2: 7-N 3 - Preparation of BFA(II)
[0069]
[0070] Under nitrogen protection, add 10mL N,N-dimethylformamide to a 50mL round-bottomed flask with a magnetic stirrer, then add compound VI 7-O-methylsulfonyl-BFA (400mg, 1.12mmol), and then add Sodium azide (217mg, 3.35mmol) was stirred at reflux at 70°C for 4h to terminate the reaction. After the reaction solution was cooled and concentrated, 30ml of ethyl acetate was added to dilute, washed with 5% citric acid solution (2×10ml), saturated sodium bicarbonate solution (2×10ml), saturated sodium chloride solution (2×10ml), and combined The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P volume ratio=1:5 to obtain compound II (Rf=0.3, yield 83.40%).
[0071] Compound Characterization:
[0072] Compound II: pale yellow solid, yield 83.4% 1 ...
Embodiment 3
[0073] Example 3: 7-NH 2 - Preparation of BFAIII
[0074]
[0075] Under the protection of helium, add 20mL tetrahydrofuran to a 50mL round bottom flask with a magnetic stirrer, then add 7-N3-BFA (305mg, 1.0mmol), triphenylphosphine (340mg, 1.3mmol) and stir at 25°C 24h to terminate the reaction. After cooling and concentrating the reaction solution, add 30ml of ethyl acetate to dilute, wash with water (2×10ml), wash with saturated sodium chloride solution (2×10ml), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to obtain the crude product. The crude product was separated by thin layer chromatography under the condition of developer D / M (volume ratio)=5:1 to obtain compound III (Rf=0.3, yield 54.80%).
[0076] Compound Characterization:
[0077] Compound III 7-NH 2 -BFA: pale yellow solid, yield 54.8% 1 H NMR (500MHz, CDCl 3 )δ7.38(dd, J=15.6,3.0Hz,1H),5.89(dd,J=15.6,1.6Hz,1H),5.72(m,1H),5.17(dd,J=15.1,9.6Hz,1H ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com