Air catalytic oxidation hydrogen bromide method for preparing 2,3,5-tribromo thiophene
A technology of catalytic oxidation and tribromothiophene, which is applied in organic chemistry and other fields, can solve problems such as the utilization rate of bromine, and achieve the effects of low production cost, low environmental pollution, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
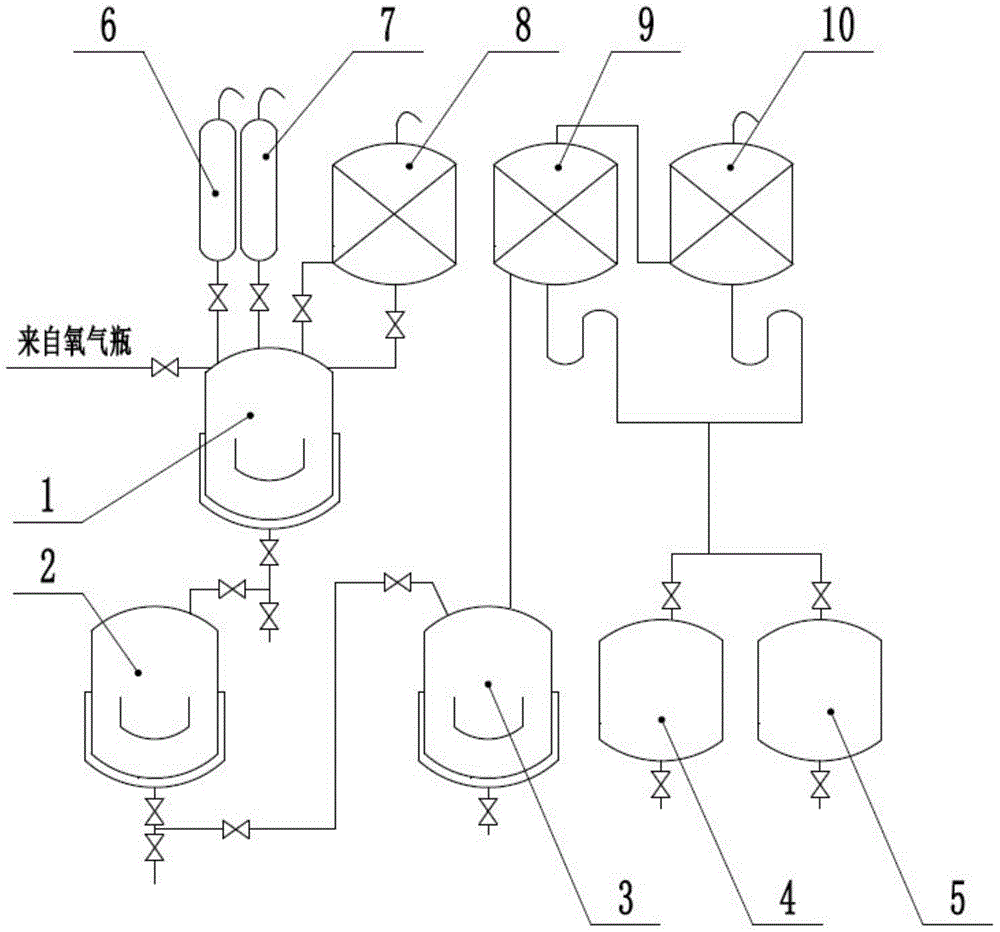
[0014] a. Put 35L of chloroform, 16.8kg of thiophene and 0.168kg of copper bromide into reactor 1, and stir for 10 minutes. When reactor 1 was cooled to -5°C with jacket water, 65.0kg of bromine was added dropwise from drop tank I6. into Reactor 1.
[0015] B. After the reaction starts, air or pure oxygen is blown into the reactor 1. After the bromine is slowly added dropwise, the reactor 1 is slowly heated to reflux. The gas is refluxed into the reactor 1 after being condensed by the reflux condenser 8, and the reaction After 3.5 hours, cool to 23°C-25°C;
[0016] c. Add 10 L of 0.05 mol / L ethanol solution of sodium hydroxide dropwise to Reactor 1 from dropping tank II7 to decompose unreacted bromine, and stir for 30 minutes.
[0017] d. Put the reaction mixture into the water washing kettle 2, add 35L of water, fully stir for 30 minutes, let it stand, and put the lower organic phase into the distillation kettle 3 for atmospheric distillation, and the gas passes through the ...
Embodiment 2
[0019] a. Put 35L of chloroform, 16.8kg of thiophene and 0.168kg of sodium nitrite into the reaction kettle 1, and stir for 10 minutes. When the reaction kettle 1 was cooled to -5°C with the jacket water, 65.0kg of bromine was added dropwise from the dropping tank I6. into Reactor 1.
[0020] B. After the reaction starts, air or pure oxygen is blown into the reactor 1. After the bromine is slowly added dropwise, the reactor 1 is slowly heated to reflux. The gas is refluxed into the reactor 1 after being condensed by the reflux condenser 8, and the reaction After 5 hours, cool to 23°C-25°C;
[0021] c. Add 10 L of 0.05 mol / L ethanol solution of sodium hydroxide dropwise to Reactor 1 from dropping tank II7 to decompose unreacted bromine, and stir for 30 minutes.
[0022] d. Put the reaction mixture into the water washing kettle 2, add 35L of water, fully stir for 30 minutes, let it stand, and put the lower organic phase into the distillation kettle 3 for atmospheric distillatio...
Embodiment 3
[0024] a. Put 35L of chloroform, 16.8kg of thiophene and 0.168kg of ferric bromide into the reaction kettle 1, and stir for 10 minutes. When the reaction kettle 1 was cooled to -5°C with the jacket water, 65.0kg of bromine was added dropwise from the dropping tank I6. into Reactor 1.
[0025] B. After the reaction starts, air or pure oxygen is blown into the reactor 1. After the bromine is slowly added dropwise, the reactor 1 is slowly heated to reflux. The gas is refluxed into the reactor 1 after being condensed by the reflux condenser 8, and the reaction After 3.5 hours, cool to 23°C-25°C;
[0026] c. Add 10 L of 0.05 mol / L ethanol solution of sodium hydroxide dropwise to Reactor 1 from dropping tank II7 to decompose unreacted bromine, and stir for 30 minutes.
[0027] d. Put the reaction mixture into the water washing kettle 2, add 35L of water, fully stir for 30 minutes, let it stand, and put the lower organic phase into the distillation kettle 3 for atmospheric distillat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com