Block copolymer anion-exchange membrane for fuel battery and preparation method of block copolymer anion-exchange membrane
An anion exchange membrane and block copolymer technology, which is used in fuel cell parts, fuel cells, solid electrolyte fuel cells, etc., can solve the problem of increased water content and electrical conductivity, decreased fuel cell performance, and poor anti-swelling performance. and other problems, to achieve the effect of enhancing water retention capacity, enhancing anti-swelling performance, and controllable degree of bromination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
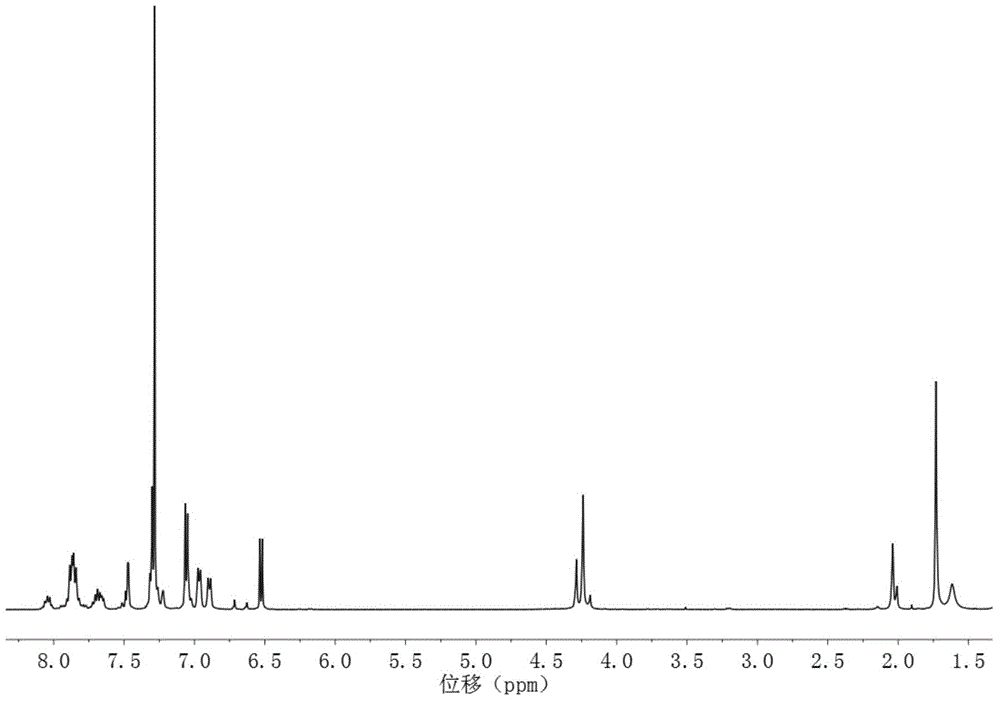
[0032] In this example, the synthesis of a block polyarylether nitrile sulfone anion exchange membrane with 20 hydrophilic segments and 20 hydrophobic segments is taken as an example. The structural formula is as follows, and the specific preparation method includes the following steps:
[0033]
[0034] Among them, m=20, n=20, R is H or
[0035] 1) Synthesis of the hydrophilic segment: 7.489g (20mmol) (ie m=20) of 3,3-bis(4-hydroxy-3,5-dimethylphenyl)phthalide, 5.334g (21mmol) (i.e. m+1=21) 4,4'-difluorodiphenyl sulfone, 4.789g of anhydrous potassium carbonate and some toluene were dissolved in N,N-dimethylacetamide, under nitrogen protection, and reacted at 145°C After 4.5h, the temperature was raised to 170°C for 12h, then 0.267g (1.05mmol) (5% (m+1)) of 4,4'-difluorodiphenylsulfone was added, and the reaction was continued at 170°C for 1h, cooled Afterwards, it is precipitated with aqueous methanol (the volume ratio of methanol to water is 1 / 1), filtered, washed, and...
Embodiment 2
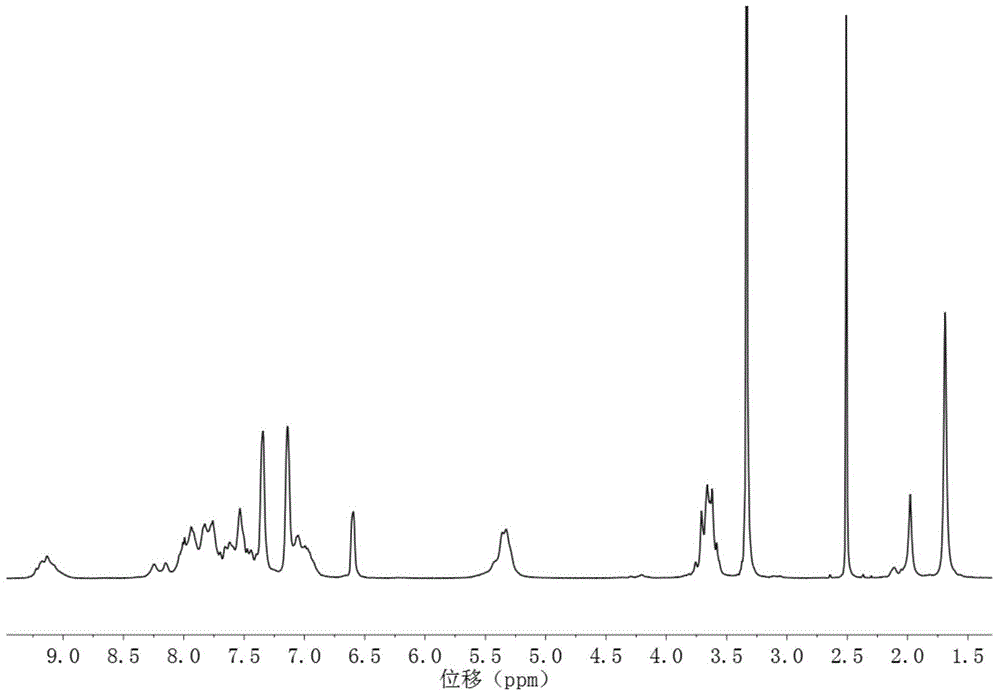
[0043] In this example, the synthesis of a block polyarylether nitrile sulfone anion exchange membrane with 10 hydrophilic segments and 20 hydrophobic segments is taken as an example. The structural formula is as follows, and the specific preparation method includes the following steps:
[0044]
[0045] Among them, m=10, n=20, R is H or
[0046] 1) Synthesis of the hydrophilic segment: 3.745g (10mmol) (ie m=10) of 3,3-bis(4-hydroxy-3,5-dimethylphenyl)phthalide, 2.794g (11mmol) (i.e. m+1=11) 4,4'-difluorodiphenyl sulfone, 2.395g of anhydrous potassium carbonate and some toluene were dissolved in N,N-dimethylacetamide, under nitrogen protection, and reacted at 145°C After 4.5h, the temperature was raised to 165°C for 12h, then 0.134g (0.55mmol) (5% (m+1)) of 4,4'-difluorodiphenylsulfone was added, and the reaction was continued at 165°C for 1h, cooled Afterwards, it is precipitated with aqueous methanol (the volume ratio of methanol to water is 1 / 1), filtered, washed, and...
Embodiment 3
[0054] This example takes the synthesis of guanidine functionalized block polyarylether nitrile sulfone anion exchange membrane as an example, the structural formula is as follows:
[0055]
[0056] Among them, m=20, n=20, R is H or
[0057] Except for the following features, other detailed steps and testing methods of the preparation method in this embodiment are the same as in Example 1.
[0058] 1) Synthesis of the hydrophilic segment: the reactant is 7.489g (20mmol) (i.e. m=20) of 3,3-bis(4-hydroxyl-3,5-dimethylphenyl)phthalide, 3.994g ( 21mmol) (ie m+1=21) of 3,3'-difluorobiphenyl, 3.456g of anhydrous potassium carbonate, and the polar aprotic solvent is N-methylpyrrolidone.
[0059] 2) Synthesis of the hydrophobic segment: the reactants are 2.782g (20mmol) (ie n=20) of 2,6-difluorobenzonitrile, 5.256g (21mmol) (ie n+1=21) of 4,4' - Sulfonyldiphenol, 4.146 g of anhydrous potassium carbonate, the polar aprotic solvent being N-methylpyrrolidone.
[0060] 3) Synthesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com