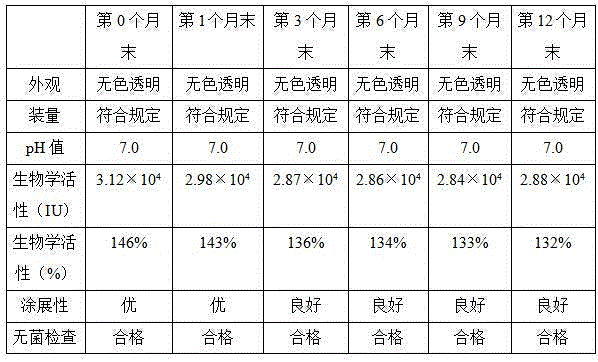
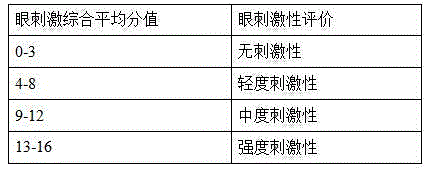
Recombinant calf alkaline fibroblast growth factor ophthalmic gel
A fibroblast and growth factor technology, applied in the field of recombinant bovine basic fibroblast growth factor ophthalmic gel and its preparation, can solve the problem of affecting the spreadability of ophthalmic gel, unfavorable use of clinical volunteers, and unfavorable Suitable for high-temperature sterilization and other issues, to achieve good biocompatibility, high safety, and long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 、 Preparation of recombinant bovine basic fibroblast growth factor ophthalmic gel of the present invention
[0038] prescription:
[0039] Recombinant bovine basic fibroblast growth factor 1680000 IU;
[0040] Human albumin 0.1 g;
[0041] Carbomer 0.8 g;
[0042] An appropriate amount of triethanolamine;
[0043] Add water for injection to 400 ml ;
[0044] A total of 1000 pieces
[0045] The preparation process is as follows:
[0046] (1) Pour water for injection into a stainless steel bucket, add the prescribed amount of carbomer under stirring, swell overnight, and obtain carbomer solution for later use;
[0047] (2) Add the prescribed amount of triethanolamine to the carbomer solution obtained in step (1), adjust the pH value to 6.5-7.5, stir evenly, and pour into the diluted tank;
[0048] (3) Add an appropriate amount of water for injection to rinse the stainless steel barrel, pour the rinsed injection water into the dilution tank, ster...
Embodiment 2
[0052] Example 2 、 Preparation of recombinant bovine basic fibroblast growth factor ophthalmic gel of the present invention
[0053] prescription:
[0054] Recombinant bovine basic fibroblast growth factor 1,500,000 IU;
[0055] Human albumin 0.05 g;
[0056] Carbomer 0.6 g;
[0057] Sodium hydroxide appropriate amount;
[0058] Add water for injection to 400 ml ;
[0059] A total of 1000 pieces
[0060] The preparation process is as in Example 1.
Embodiment 3
[0061] Example 3 、 Preparation of recombinant bovine basic fibroblast growth factor ophthalmic gel of the present invention
[0062] prescription:
[0063] Recombinant bovine basic fibroblast growth factor 1,800,000 IU;
[0064] Human albumin 0.2g;
[0065] Carbomer 1.0 g
[0066] An appropriate amount of triethanolamine;
[0067] Add water for injection to 400 ml ;
[0068] A total of 1000 pieces
[0069] The preparation process is as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com