A kind of bortezomib freeze-dried powder for injection and preparation method thereof
A technology of bortezomib and freeze-dried powder for injection is applied in the field of bortezomib freeze-dried powder for injection and its preparation, and can solve the problems of increased substance, increased medication risk, excessive moisture and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
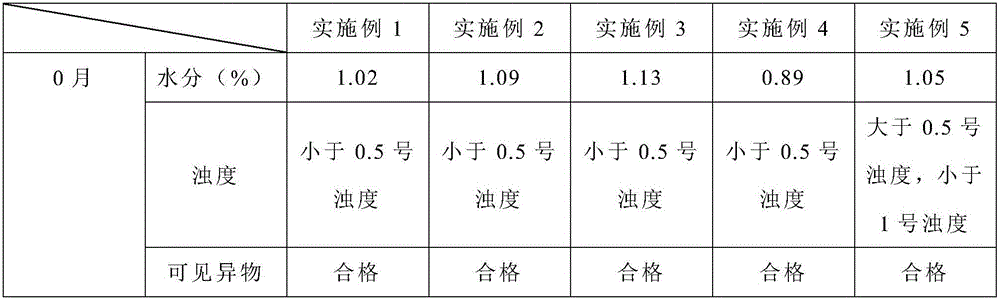
Embodiment 1
[0022] (1) Add 2.4L of water for injection into the dispensing tank, keep the water temperature at 45°C, and pass nitrogen into it for 30 minutes; add 3.5g of bortezomib, and use a homogenizer to shear at a high speed (9000 rpm) to the liquid clarify;
[0023] (2) Add 35g of mannitol to the above liquid, stir to dissolve, use 0.1mol / L hydrochloric acid solution to adjust the pH value to 5.0, add water for injection to 3.5L, and fill the liquid in In a vial, add a rubber stopper halfway (the rubber stopper is dried at 105°C for 4 hours), and put it into a lyophilizer for lyophilization.
[0024] (3) The freeze-drying curve is: before the product is put in, the shelf is cooled to -40°C in advance, and the shelf temperature is kept at -40°C for 2 hours after the product is put in; the vacuum pump is started, and the vacuum is drawn to 20Pa; the temperature is slowly raised to -5 °C and maintained for 10 hours; slowly raised to 40 °C and maintained for 4 hours.
[0025] (4) Afte...
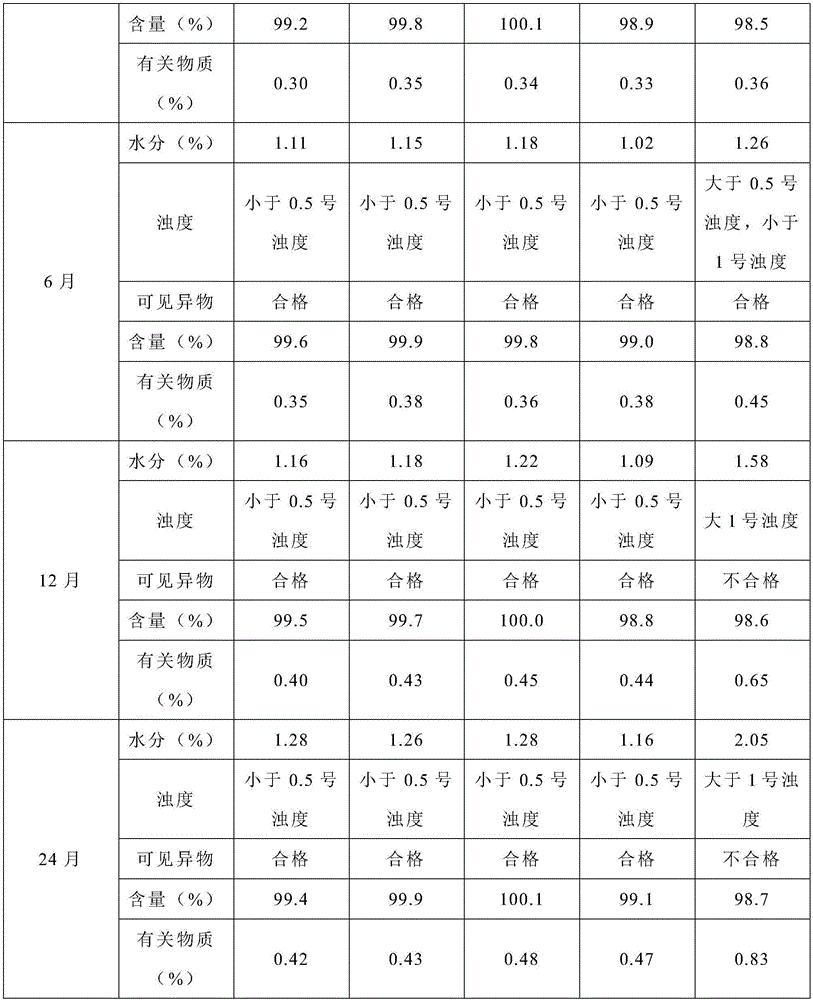
Embodiment 2
[0027] (1) Add 2.8L water for injection into the dispensing tank, keep the water temperature at 35°C, and pass carbon dioxide gas into it for 40 minutes; liquid clarification;
[0028] (2) Add 35g of mannitol to the above liquid, stir to dissolve, use 0.1mol / L hydrochloric acid solution to adjust the pH value to 3.0, add water for injection to 3.5L, and fill the liquid in In a vial, add a rubber stopper halfway (the rubber stopper is dried at 110°C for 3 hours), and put it into a lyophilizer for lyophilization.
[0029] (3) The freeze-drying curve is: before the product is placed, the shelf is cooled to -40°C in advance, and the shelf temperature is kept at -40°C for 4 hours after the product is placed; the vacuum pump is started, and the vacuum is drawn to 20Pa; the temperature is slowly raised to -5 °C and maintained for 15 hours; slowly raised to 40 °C and maintained for 6 hours.
[0030] (4) After the freeze-drying is completed, fill the freeze-drying box with nitrogen t...
Embodiment 3
[0032] (1) Add 2.6L of water for injection into the dispensing tank, keep the water temperature at 30°C, and pass argon into it for 50 minutes; add 3.5g of bortezomib, and use a homogenizer to shear at a high speed (16000 rpm) to the liquid clarification;
[0033] (2) Add 35g of mannitol to the above liquid, stir to dissolve, use 0.1mol / L hydrochloric acid solution to adjust the pH value to 4.5, add water for injection to 3.5L, and fill the liquid in In a vial, add a rubber stopper halfway (the rubber stopper is dried at 120°C for 2 hours), and put it into a lyophilizer for lyophilization.
[0034] (3) The freeze-drying curve is: before the product is placed, the shelf is cooled to -40°C in advance, and the shelf temperature is kept at -40°C for 3 hours after the product is placed; the vacuum pump is started, and the vacuum is drawn to 20Pa; the temperature is slowly raised to -5 °C and maintained for 15 hours; slowly raised to 40 °C and maintained for 6 hours.
[0035] (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com