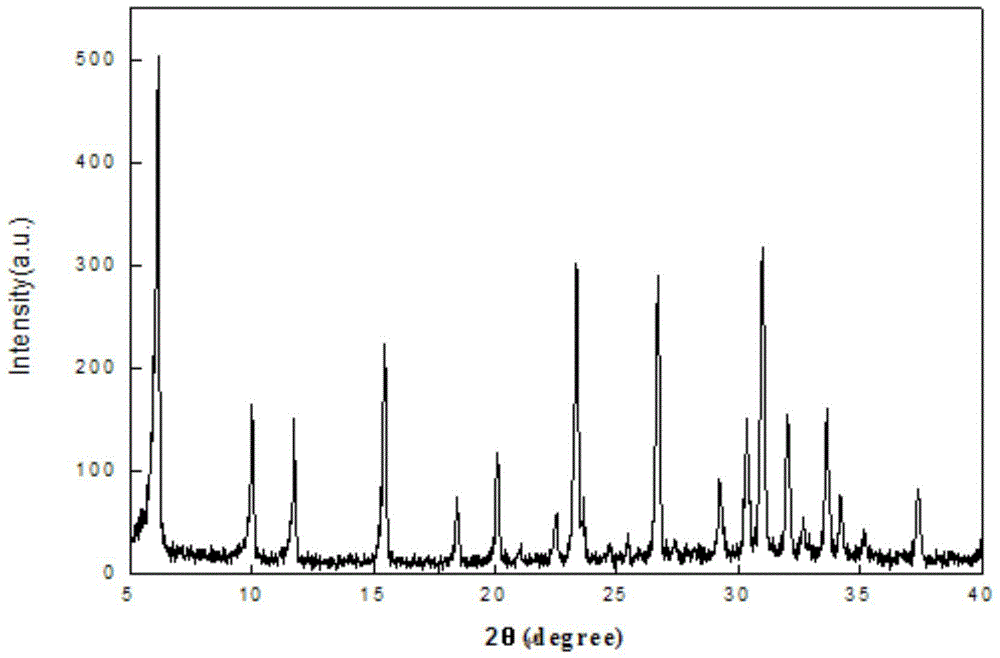
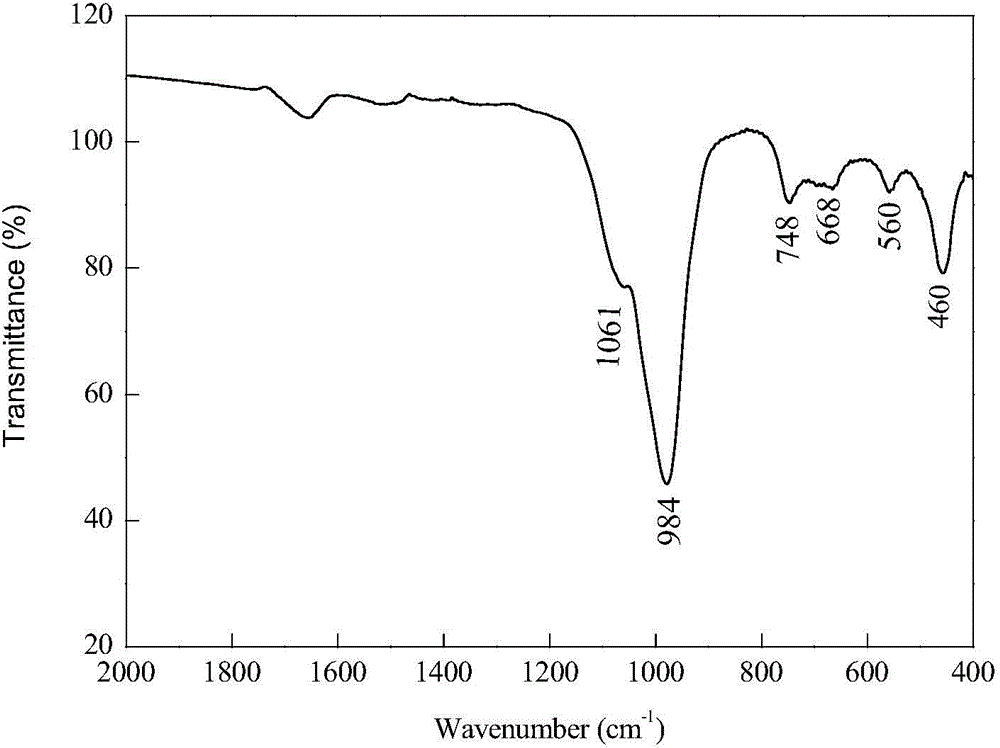
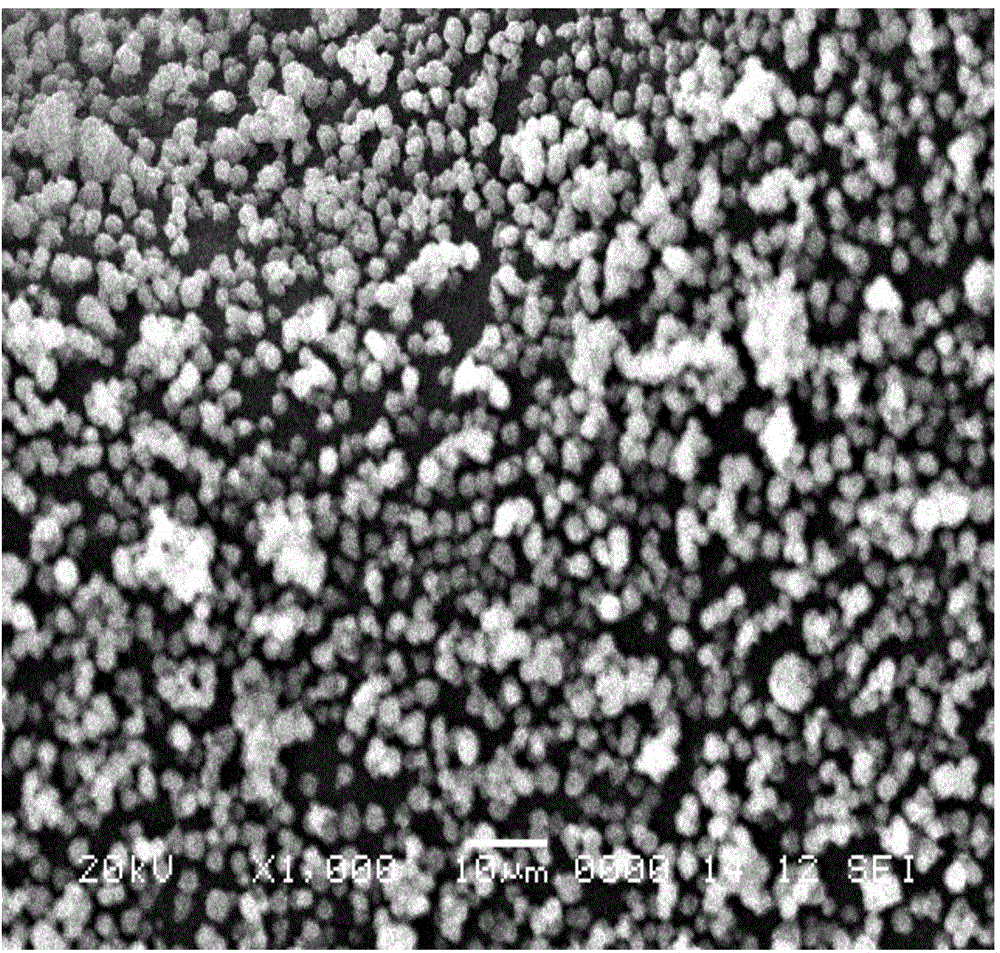
Method for preparing 13X molecular sieve by adopting coal slime
A molecular sieve and coal slime technology, which is applied in the directions of favhedral crystalline aluminosilicate zeolite, crystalline aluminosilicate zeolite, etc., to achieve the effect of improving reaction activity, reducing content and being easy to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The coal slime used in this embodiment is the coal slime produced in the coal washing process of Huangling No. 1 Mine, and the method for preparing 13X type molecular sieve by using coal slime comprises the following steps:
[0039] Step 1. Calcination and activation: Grind the coal slime and pass it through a 200-mesh sieve, then place the sieved undersize in an ash dish and spread it evenly, calcinate at 800°C for 2.5 hours, and cool naturally to obtain coal slime ash; after testing, The chemical composition of described coal slime ash is shown in Table 1:
[0040] The main chemical composition (wt%) of table 1 coal slime ash
[0041]
[0042] Step 2, acid leaching to remove impurities: mix 30mL of hydrochloric acid solution with a concentration of 6mol / L and 10.00g of the coal slime ash described in step 1, stir and react at 95°C for 4.5h, after the reaction is completed, filter while hot to obtain The filter residue, the filter residue is dried for later use; th...
Embodiment 2
[0062] The coal slime used in this embodiment is the coal slime produced in the coal washing and processing process of Yan'an Checun Coal Mine. The method for preparing 13X molecular sieves from the coal slime comprises the following steps:
[0063] Step 1. Calcination and activation: Grind the coal slime and pass it through a 200-mesh sieve, then place the sieved undersize in an ash dish and spread it evenly, calcinate at 700°C for 3 hours, and cool naturally to obtain coal slime ash; after testing, the The chemical composition of the coal slime ash is shown in Table 5:
[0064] The main chemical composition (wt%) of table 5 coal slime ash
[0065]
[0066] Step 2. Acid leaching to remove impurities: Mix 80 mL of hydrochloric acid solution with a concentration of 5 mol / L and 10.00 g of the coal slime ash described in Step 1, and stir and react at 90°C for 5 hours. After the reaction is completed, filter while hot to obtain a filter residue , the filter residue is dried fo...
Embodiment 3
[0084] The coal slime used in this embodiment is the coal slime produced in the coal washing and processing process of Huangling No. 2 Mine, and the method for preparing 13X type molecular sieve by using coal slime comprises the following steps:
[0085] Step 1. Calcination and activation: Grind the coal slime and pass it through a 200-mesh sieve, then place the sieved undersize in an ash dish and spread it evenly, calcinate at 750°C for 2 hours, and cool naturally to obtain coal slime ash; after testing, the The chemical composition of coal slime ash is shown in Table 9:
[0086] The main chemical composition (wt%) of table 9 coal slime ash
[0087]
[0088] Step 2, acid leaching to remove impurities: mix 70mL of hydrochloric acid solution with a concentration of 5.5mol / L and 10.00g of coal mud ash described in step 1, stir and react at 110°C for 3h, after the reaction is completed, filter while hot to obtain The filter residue, the filter residue is dried for later use; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com