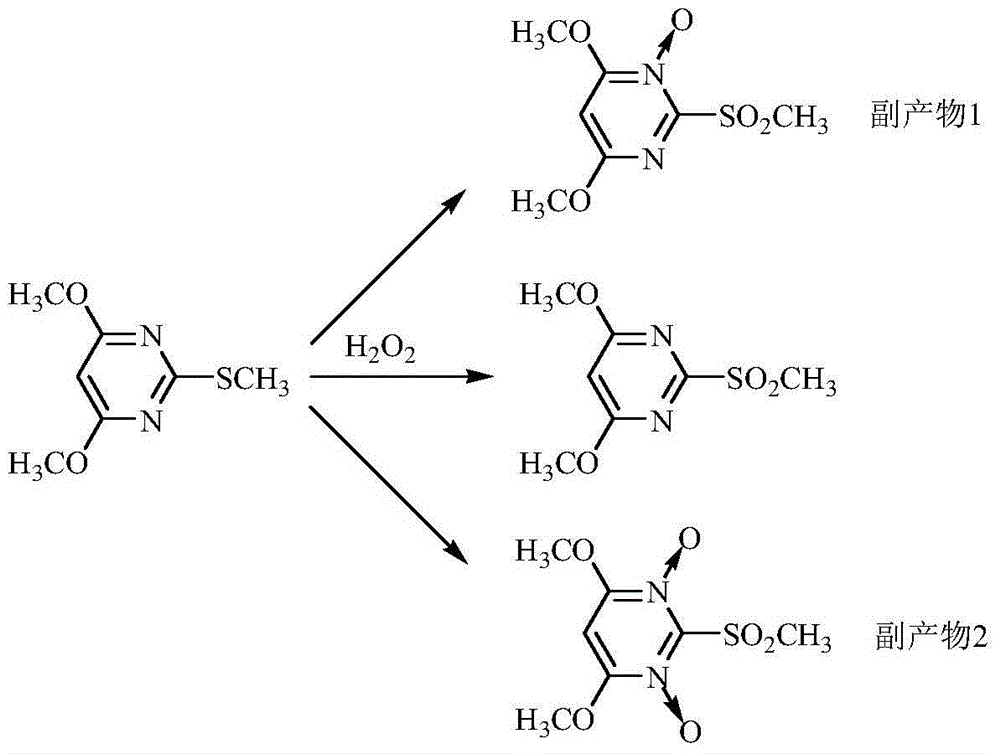
Preparation method of bispyribac-sodium intermediate 4,6-dimethoxy-2-melthyl sulfonyl pyrimidine
A technology of methanesulfonylpyrimidine and dimethoxy is applied in the field of efficient preparation of bispyribac intermediate 4,6-dimethoxy-2-methanesulfonylpyrimidine, and can solve the problems of low oxidation efficiency and environmental pollution Difficult to overcome, catalyst can not be recovered and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 10g template triblock surfactant P123 in 500mL 5% HCl solution, stir at high speed,
[0026] Add 37g of aluminum isopropoxide and 325g of ethyl orthosilicate, hydrolyze to prepare a transparent sol, put it into a polytetrafluoroethylene-lined autoclave, crystallize, filter, wash, and dry. The muffle furnace was programmed to raise the temperature at 2 °C / min to 200 °C for 2 h, and at 5 °C / min to 500 °C for 5 h to obtain the Al-SBA-15 catalyst carrier.
[0027] Dissolve 10g of phosphotungstic acid in 30g of water, add 100g of Al-SBA-15 catalyst, impregnate for 12h, dehydrate by rotary evaporation, dry at 120°C for 5h, and roast at 300°C for 5h in a muffle furnace to obtain the supported catalyst of phosphotungstic acid.
[0028] Add 930g acetic acid, 186g 4,6-dimethoxy-2-methylthiopyrimidine, and 18.6g phosphotungstic acid supported catalyst in the reactor, raise the temperature to 50°C, slowly add 340g 30wt% hydrogen peroxide dropwise, TLC tracking After the r...
Embodiment 2
[0030] Dissolve 15g of template triblock surfactant P123 in 600mL of 5% HCl solution, add 225g of aluminum isopropoxide and 242g of tetraethyl orthosilicate under high-speed stirring, hydrolyze to prepare a transparent sol, and put it into a polytetrafluoroethylene liner Autoclave, crystallization, filtration, washing and drying. The muffle furnace was programmed to raise the temperature at 2 °C / min to 200 °C for 2 h, and at 5 °C / min to 500 °C for 5 h to obtain the Al-SBA-15 catalyst carrier.
[0031] Dissolve 5g of phosphomolybdic acid in 20g of water, add 100g of Al-SBA-15 catalyst, impregnate for 12h, spin-evaporate and dehydrate, dry at 120°C for 5h, and roast at 300°C for 5h in a muffle furnace to obtain a phosphomolybdic acid-supported catalyst.
[0032] In the reactor, add 1860g acetic acid, 186g 4,6-dimethoxy-2-methylthiopyrimidine, and 93g phosphomolybdic acid supported catalyst, raise the temperature to 60°C, slowly add 1133g 30wt% hydrogen peroxide dropwise, TLC tra...
Embodiment 3
[0034] Dissolve 11g template tri-block surfactant P123 in 500mL 5% HCl solution, under high-speed stirring, add 76g aluminum isopropoxide and 312g tetraethyl orthosilicate, hydrolyze to prepare a transparent sol, and put it into a polytetrafluoroethylene liner Autoclave, crystallization, filtration, washing and drying. The muffle furnace was programmed to raise the temperature at 2 °C / min to 200 °C for 2 h, and at 5 °C / min to 500 °C for 5 h to obtain the Al-SBA-15 catalyst carrier.
[0035] Dissolve 30g of silicotungstic acid in 50g of water, add 100g of Al-SBA-15 catalyst, impregnate for 12h, spin dehydrate, dry at 120°C for 5h, and bake at 300°C for 5h in a muffle furnace to obtain the supported catalyst of silicotungstic acid.
[0036] Add 1488g acetic acid, 186g 4,6-dimethoxy-2-methylthiopyrimidine, and 55.8g silicotungstic acid supported catalyst into the reactor, raise the temperature to 80°C, slowly add 680g 30wt% hydrogen peroxide dropwise, TLC tracking After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com