Method for preparing gamma-valerolactone from furfural on metal/solid acid catalyst
A technology of solid acid catalyst and valerolactone, which is applied in the direction of organic chemistry, can solve the problems of high energy consumption, unfavorable large-scale production, and influence on product selectivity, and achieve low cost, easy operation, and reduced human operation loss. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] Catalyst preparation
[0019] Dissolve a calculated amount of metal salt solution in a small amount of water, and prepare metal / solid acid catalysts in three ways: (1) load the metal salt solution on the solid acid by impregnation method, (2) pre-coat the metal solution (3) Titrate the metal hydroxide precipitate with an alkali solution, then bake it into a metal oxide, and mechanically mix it with the solid acid. Roasted at high temperature, then reduced with hydrogen. Among them, the silicon-aluminum molecular sieve and the phosphorus-aluminum molecular sieve in the solid acid are commercial products, the metal oxide and phosphate are prepared by precipitation of corresponding metal precursors, and the metal sulfate is prepared by impregnating the corresponding metal oxide with sulfuric acid.
Embodiment 1
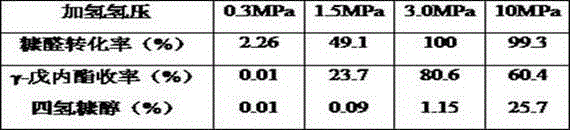
[0021] Add 0.15g of furfural and 5g of solvent into a batch-type high-pressure reactor with a polytetrafluoroethylene liner, and add catalysts in sequence. At the initial hydrogen pressure of 3MPa, 120 o Magnetic high-speed stirring reaction at C temperature for 6 hours; turn off hydrogen, feed nitrogen, continue to feed hydrogen after 12 hours of reaction, hydrogen pressure 3MPa, after 6 hours of reaction, separate the catalyst from the reaction solution. The reaction liquid was analyzed by gas chromatography, and the conversion rate of furfural and the yield of γ-valerolactone are shown in Table 1.
[0022] When transition metals are used as hydrogenation catalyst components, the main by-products are dark carbon deposits; when noble metals are used as catalyst hydrogenation components, the by-products are various overhydrogenation products, such as MTHF, alcohols and alkanes, etc., through Separation from the main product by means of vacuum distillation and the like.
Embodiment 2
[0023] Embodiment 2 (comparative example)
[0024] The reaction raw materials and steps are the same as in Example 1, and the catalyst used is 25wt%CoFe / ZrSO 4 , the difference is that the hydrogen pressure has been maintained at 3MPa, and nitrogen is not introduced. The conversion rate of furfural and the yield of γ-valerolactone are shown in Table 1.
[0025] Table 1 Properties of furfural to prepare γ-valerolactone over different catalysts
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com