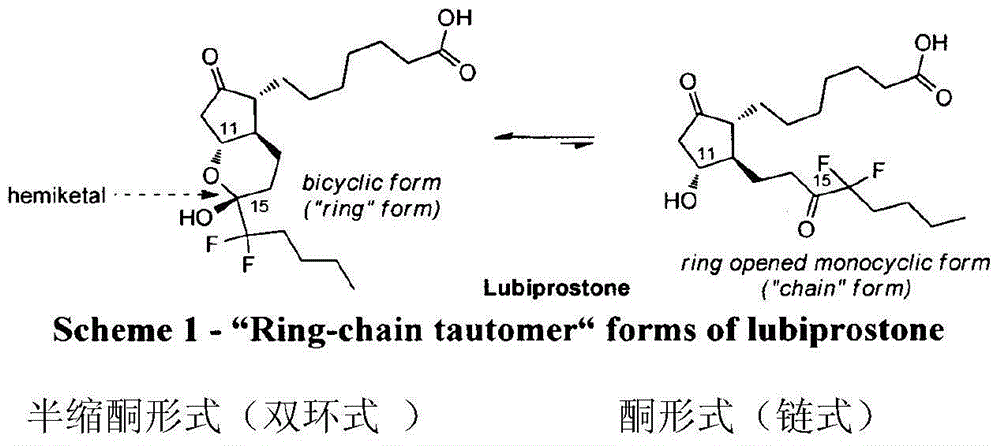
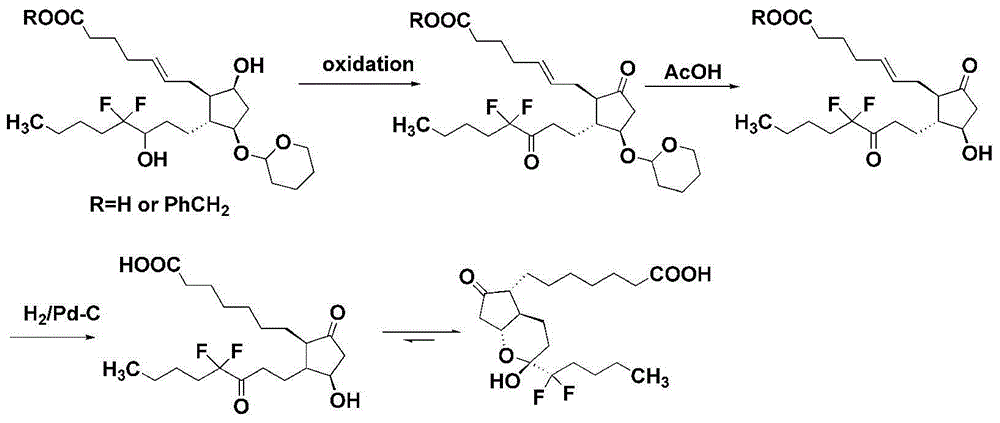
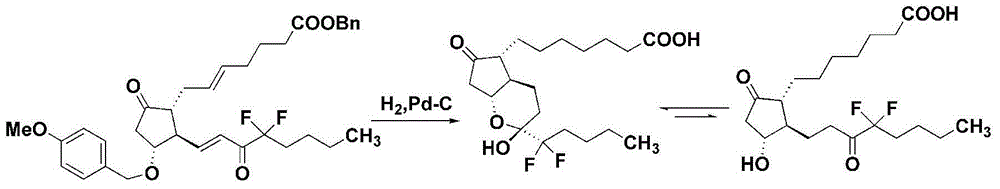
Method for preparing lubiprostone compound
A technology for lubiprostone and compounds, applied in organic chemistry, bulk chemical production, etc., can solve the problems of pressure-catalyzed hydrogenation, cumbersome reaction steps, difficult to complete conversion, etc., and achieve the improvement of reaction efficiency and purity, equipment The effect of low requirements and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] At room temperature, add 0.5g (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-3-hydroxyl-5- Oxocyclopentyl]heptanoic acid-5-enbenzyl ester and 10ml methanol, add 0.1g of 10%Pd / C (50%H 2 O), then dilute 0.7g triethylsilane with 10ml methanol to make a solution, slowly drop it into the reaction flask, after the dropwise addition, keep the temperature of 15-25°C for 10 minutes, filter to remove palladium carbon, and concentrate the reaction solution Add 20ml of ethyl acetate, wash with 20ml of saturated sodium chloride, dry the organic layer over anhydrous sodium sulfate, concentrate the filtrate and evaporate to dryness to an oily substance to obtain lubiprostone. Weighing 0.39g, yield 97.5%, product purity 98.12%.
[0046] MS(m / z):389.46(M-1).
Embodiment 2
[0048] At room temperature, add 0.5g (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-3-hydroxyl-5- Oxocyclopentyl]heptanoic acid-5-enbenzyl ester and 10ml of ethanol, add 0.1g of 10%Pd / C (50%H 2 0), then dilute 0.7g triethylsilane with 10ml ethanol to form a solution, slowly drop it into the reaction bottle, after the dropwise addition, keep the reaction at 15-25°C for 30 minutes, remove palladium carbon by filtration, and concentrate the reaction solution Add 20ml of ethyl acetate, wash with 20ml of saturated sodium chloride, dry the organic layer over anhydrous sodium sulfate, filter, remove the desiccant, concentrate the filtrate and evaporate to dryness, weigh 0.39g, yield 97.5%. The product purity is 98.05%.
Embodiment 3
[0050]0.5g (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-3-hydroxyl-5-oxocyclopentyl]heptanoic acid Add -5-enbenzyl ester into methanol, stir to dissolve completely, add palladium hydroxide under nitrogen protection, add 0.7g triethylsilane to 10mL methanol to dilute, then add dropwise to the above solution for 10-15 minutes After dropping, react at 20-25°C for 30 minutes, remove palladium hydroxide by filtration, add 20mL of ethyl acetate after concentrating the reaction solution, wash once with 20mL of saturated sodium bicarbonate solution and 20mL of saturated sodium chloride, anhydrous sulfuric acid Sodium dry. After filtering and removing the desiccant, the filtrate was concentrated to obtain lubiprostone, weighing 0.375g, with a yield of 93.8% and a product purity of 97.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com