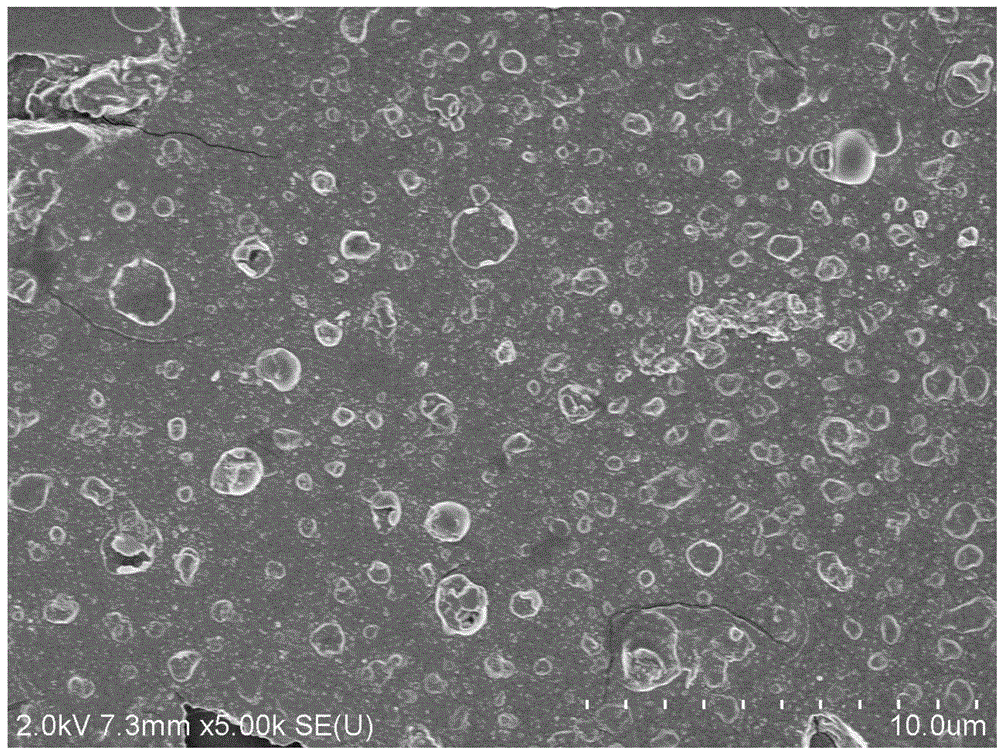
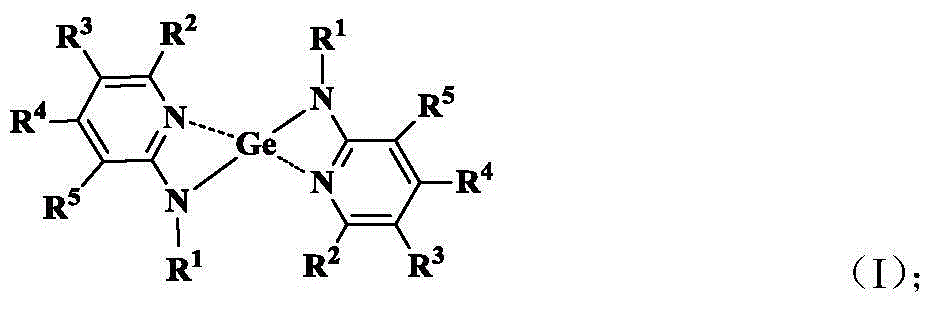
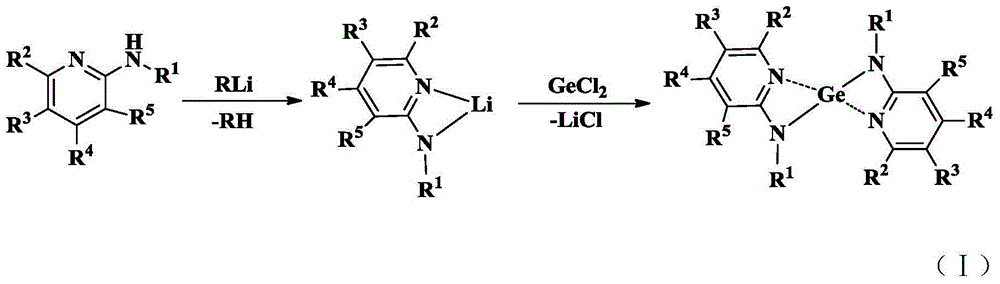
Aminopyridine Ge(II) proplastid used as microelectronic phase change memory as well as preparation method of aminopyridine Ge(II) proplastid
A phase change memory and aminopyridine technology, applied in the field of semiconductor and microelectronic materials, can solve the problems of harsh conditions, complex synthesis process, poor thermal stability, etc., and achieve the effects of mild preparation conditions, simple synthesis method and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: a preparation method of aminopyridine Ge(II) used as a microelectronic phase change memory, comprising the following steps:
[0033] (1) Dissolve 2-aminopyridine in diethyl ether, the mass ratio of 2-aminopyridine to diethyl ether is 1:5, add to the diethyl ether solution of methyl lithium under the condition of keeping stirring at -78 ° C, 2-aminopyridine and The molar ratio of methyllithium was 1.0:1.0, the concentration of the ether solution of methyllithium was 1.0M, and the stirring speed was 700 rev / min; after returning to room temperature, the stirring reaction was continued for 2 hours, and the Li complex was allowed to stand after the reaction was completed. stand-by;
[0034] (2) The Li complex obtained in step (1) is mixed with toluene, and the mass ratio of the Li complex to toluene is 1:5 to obtain a toluene solution of lithium salt. Under the condition of -78 ℃, according to the molar ratio of lithium salt and metal germanium 2.0:1.0, the to...
Embodiment 2
[0037] Embodiment 2: a preparation method of aminopyridine Ge(II) used as a microelectronic phase change memory, comprising the following steps:
[0038](1) Dissolve 2-trimethylsilylaminopyridine in n-hexane solvent, the mass ratio of 2-trimethylsilylaminopyridine to n-hexane solvent is 1:10, and add formaldehyde while stirring at -65°C In the ether solution of lithium lithium, the molar ratio of 2-trimethylsilylaminopyridine to methyl lithium is 1.0:1.2, the concentration of the ether solution of methyl lithium is 1.3M, and the stirring speed is 1300 rpm; return to room temperature Then continue to stir and react for 3 hours, after the reaction finishes, the Li complex is left standing for use;
[0039] (2) Mix the Li complex obtained in step (1) with toluene, the mass ratio of the Li complex to toluene is 1:10, to obtain a toluene solution of the lithium salt. At -65°C, according to the molar ratio of lithium salt to metal germanium of 2.0:1.2, the toluene solution of lithi...
Embodiment 3
[0042] Embodiment three: a kind of preparation method of aminopyridine Ge(II) used as microelectronic phase change memory, comprises the following steps:
[0043] (1) Dissolve 2-methylaminopyridine in n-hexane solvent, the mass ratio of 2-methylaminopyridine to n-hexane solvent is 1:15, add methyllithium ether solution under the condition of keeping stirring at -54°C , the molar ratio of 2-methylaminopyridine to methyllithium is 1.0:1.2, the concentration of the ether solution of methyllithium is 1.5M, and the stirring speed is 1700 rpm; after returning to room temperature, continue to stir and react for 4 hours, and the reaction ends Afterwards, the Li complex is left to stand for use;
[0044] (2) Mix the Li complex obtained in step (1) with toluene, and the mass ratio of the Li complex to toluene is 1:15 to obtain a toluene solution of lithium salt. At -54°C, according to the molar ratio of lithium salt to metal germanium of 2.0:1.3, the toluene solution of lithium salt wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com