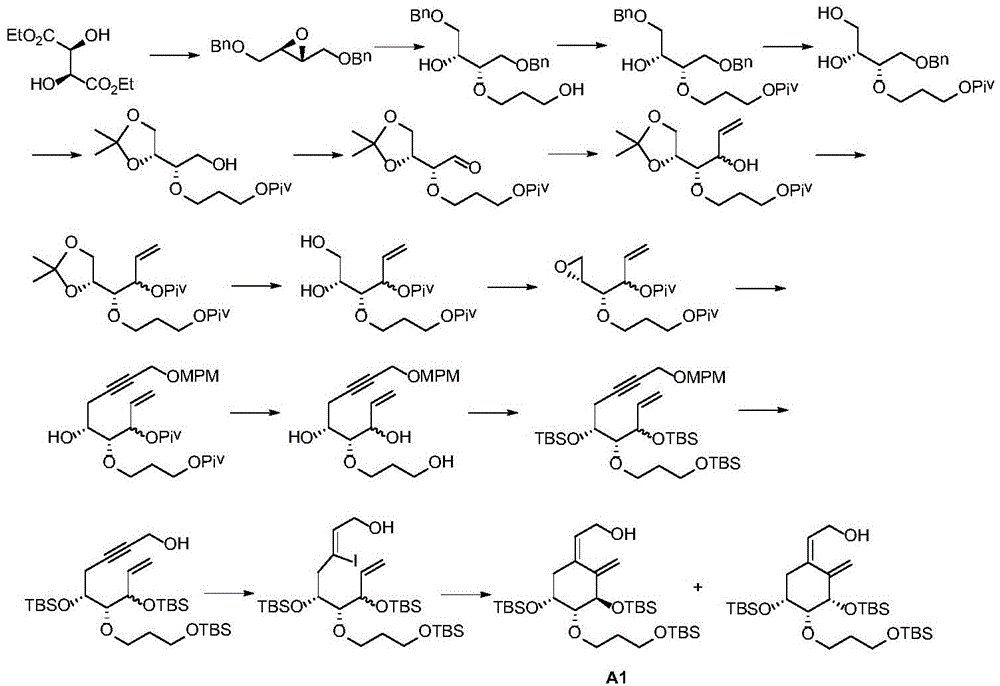
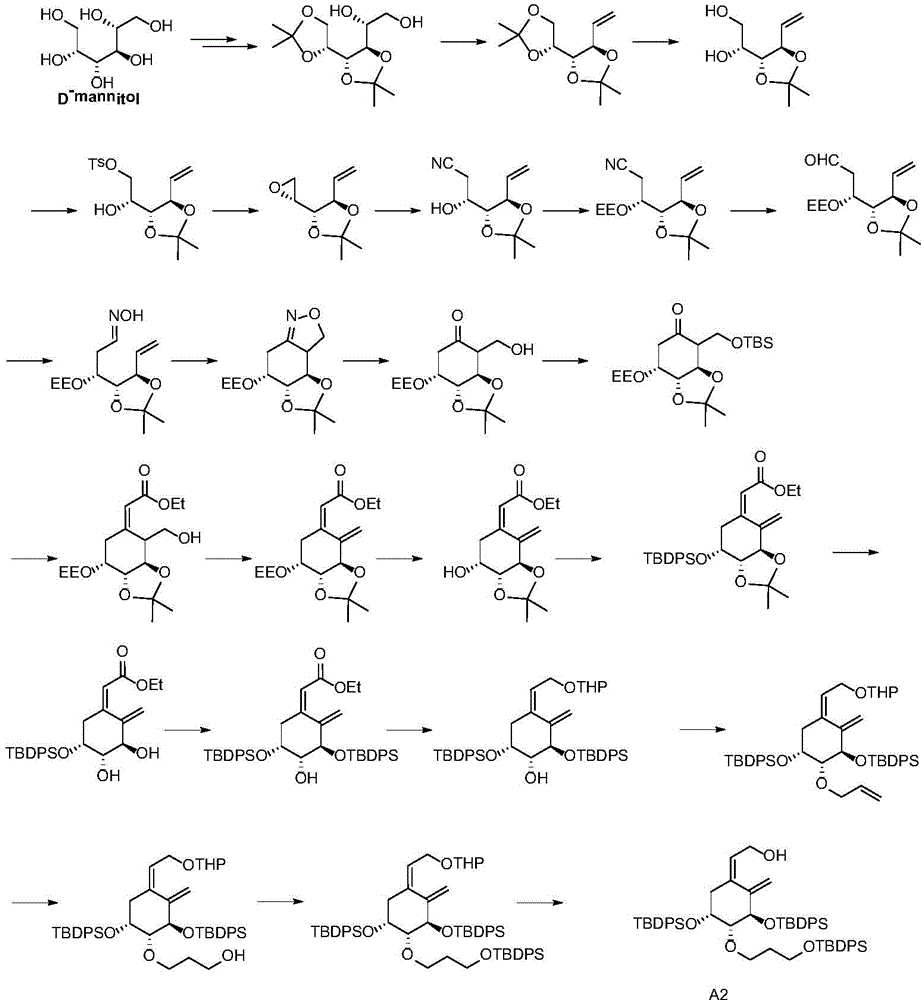
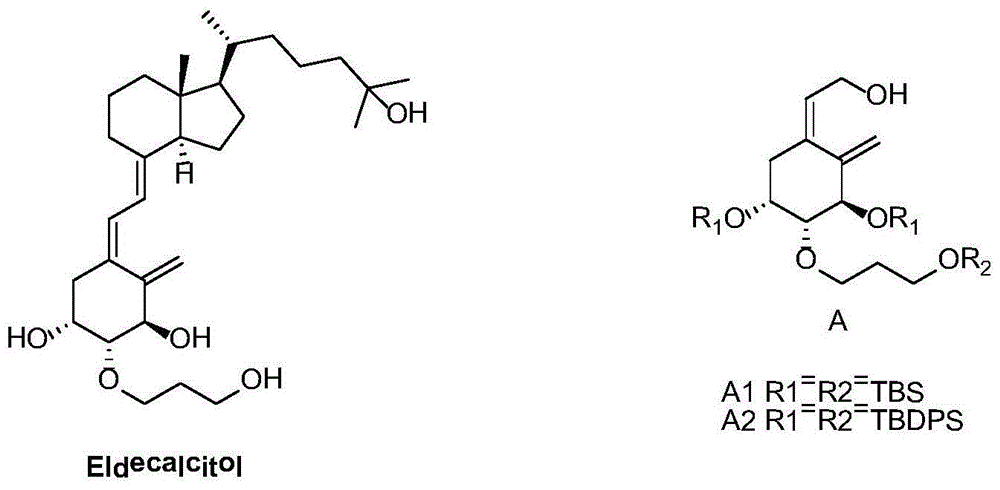
Preparation method of derivative A of (Z)-2-((3R,4R,5R)-3,5-dihydroxy-4-(3-hydroxy-propoxy)-2-methylene cyclohexyl) ethanol
A technology of dioxane and compound is applied in the field of compound preparation, and achieves the effects of reduced cost, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of compound 2
[0058]
[0059] Compound 1 (500g, 2.66mol) and imidazole (542.5g, 7.97mol) were dissolved in DMF (5.0L), and tert-butyldimethylsilyl chloride (1000g, 6.65mol) was added in portions at room temperature. Reaction 4.0h. TLC detects that the raw material spot disappears, and the reaction is complete. Add aqueous sodium bicarbonate solution to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate to give the crude product as a pale yellow oil. The crude product was chromatographed on a silica gel column to obtain 940 g of compound 2 as a light yellow oil, with a yield of 85%.
[0060] 1H NMR (400MHz, CDCl3): 6.75(s, 1H), 4.40(d, 1H, J=12.1Hz), 4.05(d, 1H, J=3.1Hz), 3.83(m, 1H), 3.75(s, 3H),2.49(dd,1H,J=14.1Hz),2.26(d,2H,J=6.1Hz),0.885(s,9H),0.855(s,9H),0.125(s,3H),0.114( s,3H),0.089(s,3H),0.0614(s,3H).LC-MS(ESI-TOF):calcd for[C 20 ...
Embodiment 2
[0061] Embodiment 2: the preparation of compound 3
[0062]
[0063] Dissolve compound 2 (300g, 721mmol) in DMF (3.0L), add potassium tert-butyl alkoxide (121g, 1.08mol) in batches under ice-water bath, stir for 0.5h under ice-water bath, add (3-bromopropyl ) tert-butyldimethylsilane (726 g, 2.88 mol). Continue to incubate for 12 hours, TLC detects that the raw material spots disappear, and the reaction is complete. Add saturated ammonium chloride aqueous solution to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate to give the crude product as a pale yellow oil. The crude product was chromatographed on a silica gel column to obtain 310 g of compound 3 as a light yellow oil, with a yield of 73%.
[0064] 1H NMR (400MHz, CDCl3): 6.85(s, 1H), 3.95(d, 1H, J=10.1Hz), 3.85(s, 1H), 3.80(s, 1H), 3.76(s, 3H), 3.71( m,4H),2.44(dd,2H,J=8.1Hz),2.26(m,2H),0.887-0.916(m,27H),0.066-0.074(m,...
Embodiment 3
[0065] Embodiment 3: the preparation of compound 4
[0066]
[0067] Compound 3 (200 g, 334 mmol) was dissolved in ethanol (2.0 L), and an aqueous solution (1.0 L) of potassium permanganate (528 g, 3.34 mol) was added dropwise at zero temperature. After the dropwise addition, keep the temperature and react until the spots of raw materials observed by TLC disappear, add water to quench, filter, extract with ethyl acetate, combine organic phases, wash with saturated NaCl, anhydrous NaCl 2 SO 4 Dry and concentrate to give a red crude product. Silica gel column chromatography gave 146 g of colorless oil 4, with a yield of 70%.
[0068] 1H NMR (400MHz, CDCl3): 4.55(d, 1H, J=6.3Hz), 4.51(s, 1H,), 4.08(s, 1H), 3.75(m, 4H), 3.62(dd, 2H, J= 9.3Hz), 3.45(t, 2H), 2.59(dd, 1H, J=4.3Hz), 2.12(t, 1H), 1.69(t, 2H, J=6.2Hz), 1.61(t, 1H), 0.86 -0.94(m,27H),0.18(d,6H,J=5.6Hz),0.061-0.10(m,12H).LC-MS(ESI-TOF):calcd for[C 29 h 62 o 8 Si 3 +H] + 623.38, found 623.38
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com