Chiral N-heterocyclic carbene precursor salt with 3,4-dihydroisoquinoline skeleton, synthetic method and application
A technology of dihydroisoquinoline and dihydroisoquinolinone, which is applied in the field of synthesis of chiral nitrogen heterocyclic carbene precursor salts, can solve the problem that catalysts cannot obtain enantioselective control, chiral control, and skeleton The structure is limited and other problems, and the starting materials are cheap and easy to obtain, suitable for mass production, and the synthesis method is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
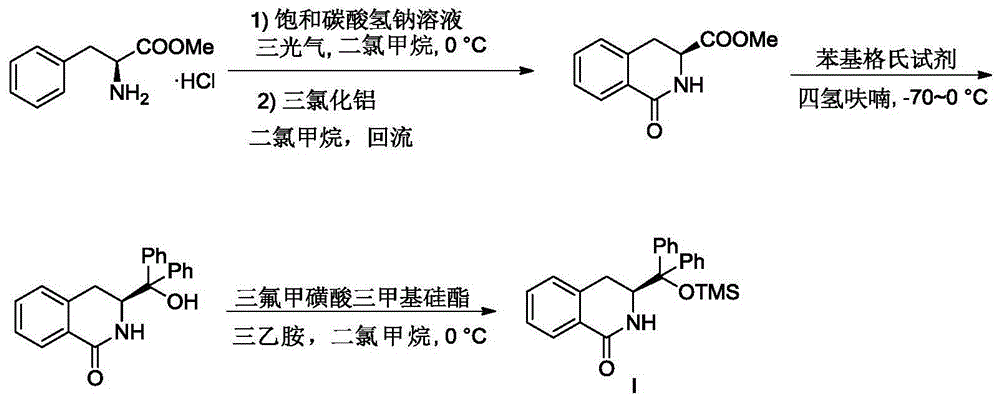
[0026] Embodiment 1: the synthesis of chiral 3,4-dihydroisoquinolinone
[0027]
[0028] At 0°C, add dichloromethane (3 mL / mmol) to phenylalanine methyl ester hydrochloride (1 equivalent) and stir, add saturated sodium bicarbonate solution (3 mL / mmol), and stir vigorously for about 15 minutes until there is no more gas release. Triphosgene (1 equiv) was added and stirred vigorously for 15 minutes. Stirring was stopped, the liquid was separated, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, the solvent was spun out under reduced pressure, drained by an oil pump, and used directly for the next reaction. Under an ice bath, the reaction product from the previous step was dissolved in dichloromethane (3 mL / mmol), slowly added anhydrous aluminum trichloride and vigorously stirred, returned to room temperature after the addition, and started reflux reaction. After the complete reaction of the ra...
Embodiment 2
[0046] Example 2: Synthesis of azacyclic carbene precursor salt of hydroxyl-protected chiral 3,4-dihydroisoquinoline skeleton
[0047]
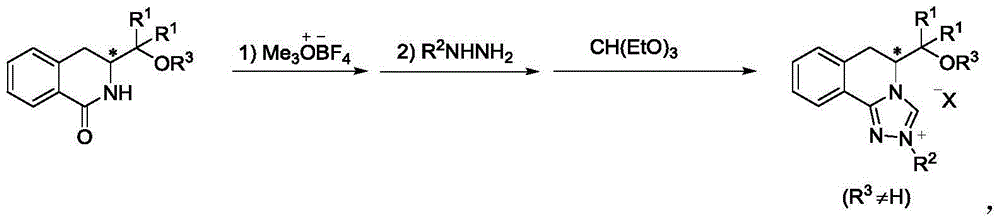
[0048] The 3,4-dihydroisoquinolinone (1 equivalent) in Example 1 was dissolved in dichloromethane (10 mL / mmol), trimethyloxytetrafluoroborate (Meerwein reagent, 1.4 equivalents) was added rapidly, 20 Reaction at ℃ for 12h. Hydrazine R 2 NHNH 2 (1.4 equivalents) continued to react at room temperature for 48h. The solvent was removed under reduced pressure, triethyl orthoformate (10 mL / mmol) was added, and the reaction was continued at 80°C. NMR monitoring showed that the raw materials were completely reacted, and the solvent was removed under reduced pressure. Preliminary purification by column chromatography and recrystallization with ethyl acetate and petroleum ether.
[0049]
[0050] 1.05g, 59% yield (3mmol), (EA / PE=1 / 4-1 / 1)[α] D 20 =-29.8 (c=0.5, CHCl 3 ), m.p.=197-198℃. 1 H NMR (300MHz, CDCl 3 )δ10.12(s,1H),7.98(d,J=7.8Hz...
Embodiment 3
[0107] Embodiment 3: the synthesis of the chiral nitrogen heterocyclic carbene precursor salt containing chlorine anion
[0108]
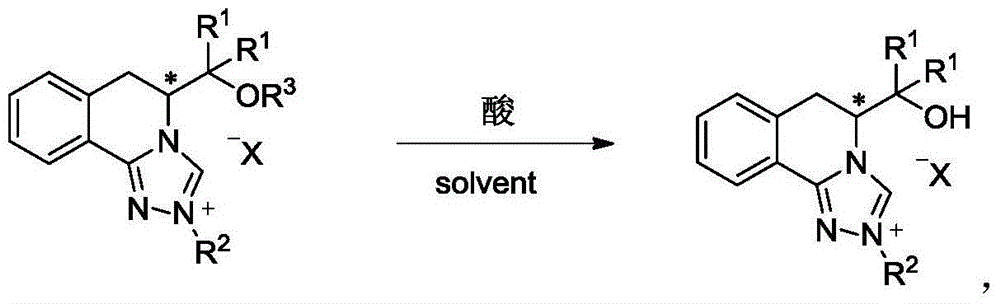
[0109] 3,4-dihydroisoquinolinone (1 equivalent) in Example 1 was dissolved in CH 2 Cl 2 (10mL / mmol), trimethyloxytetrafluoroborate (Meerwein reagent, 1.4 equivalents) was added quickly, and reacted at 20°C for 12h. The reaction was quenched by adding saturated aqueous sodium bicarbonate solution, separated, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and purified by column chromatography.
[0110] The previous step product was dissolved in dichloromethane, and the hydrochloride (R 2 NHNH 2 ) (1.4 equivalents), the reaction was continued for 48h at room temperature. The solvent was removed under reduced pressure, triethyl orthoformate (10 mL / mmol) was added, and the reaction was continued at 80°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com