Preparation method of recessive malachite green hapten
A technology of recessive malachite green and hapten, which is applied in the field of immunochemistry, can solve the problems of cumbersome synthesis steps of recessive malachite green hapten, low final yield, and reduce the impact of antibodies, so as to reduce emissions and reaction by-products The effect of less and less organic solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
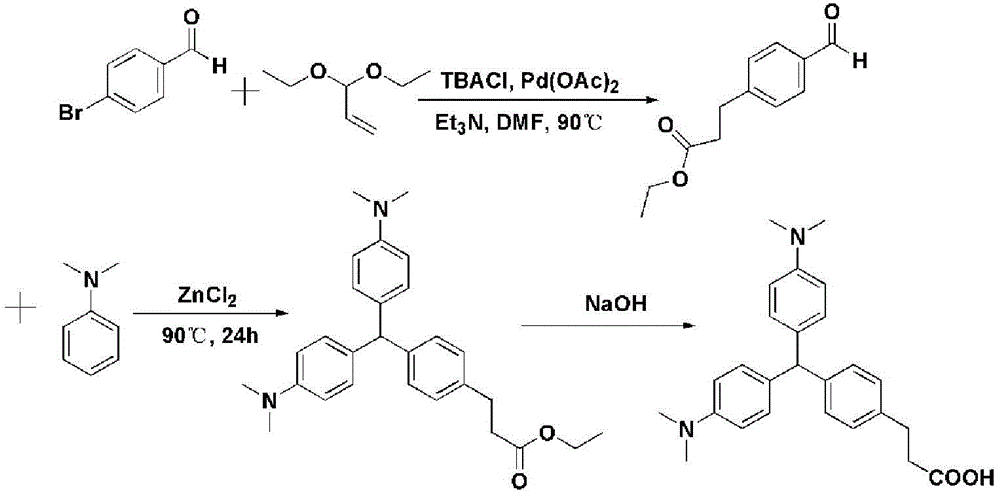
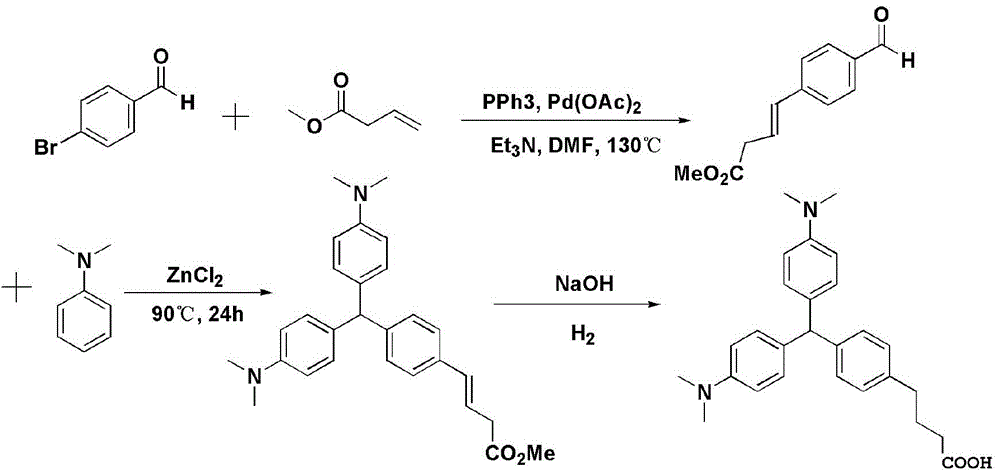
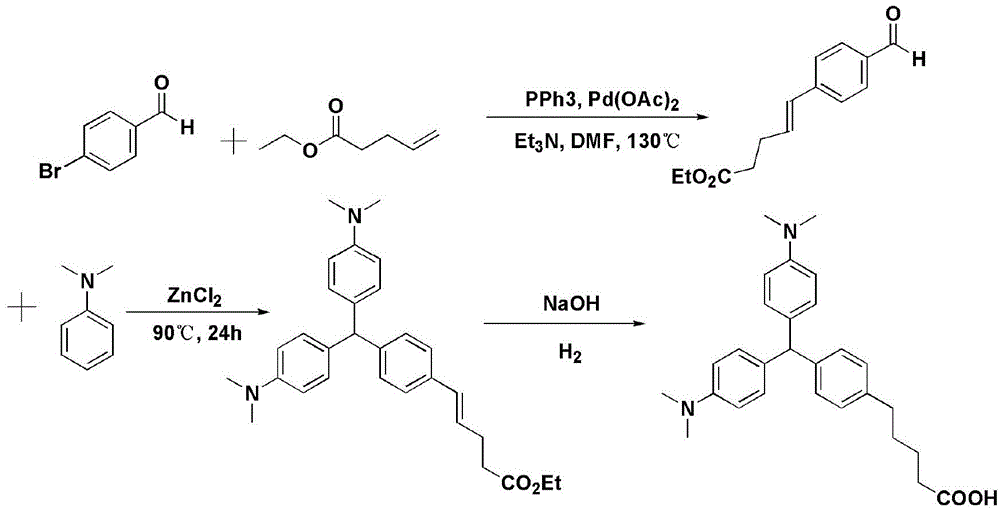
[0026] The invention provides a method for preparing a recessive malachite green hapten, the method comprising the following steps:
[0027] Step 1: carry out Heck reaction with 4-bromobenzaldehyde or 4-iodobenzaldehyde and acrolein diethyl acetal or the compound shown in general formula (I) in the presence of Heck reaction ligand, palladium acetate and acid-binding agent Compound 1 is obtained;
[0028] Step 2: Condensing compound 1 with N,N-dimethylaniline to obtain compound 2;
[0029] Step 3: dissolving compound 2 in 1,4-dioxane, adding saturated sodium hydroxide solution to a pH value of 9-10, and reacting to obtain the recessive malachite green hapten represented by general formula (II);
[0030]
[0031] Wherein, in the general formula (I), n is an integer of 1-3, and m is 0 or 1; in the general formula (II), n is an integer of 2-4.
[0032] In a preferred embodiment of the present invention, the recessive malachite green hapten is prepared by using acrolein diethy...
Embodiment 1
[0056] This example is used to illustrate the preparation method of the recessive malachite green hapten provided by the present invention.
[0057] Add 1.85g of 4-p-bromobenzaldehyde, 2.78g of tetrabutylammonium chloride and 0.067g of palladium acetate into a two-necked flask and mix well, then add N,N-dimethylformamide (DMF) under the protection of argon 40mL was used as the reaction solvent, and then 4.56mL of acrolein diethyl acetal and 2.79mL of triethylamine were added sequentially, stirred at 90°C (500rpm) for reaction, and a small amount was taken every 6 hours to judge the progress of the reaction by TLC. The reaction ended after 48h. The reaction solution was poured into 400 mL of ice water, stirred evenly, and then extracted with ethyl acetate 4 times, and the amount of ethyl acetate used each time was 150 mL. After the organic phase was dried by rotary evaporation, it was separated by column using petroleum ether: ethyl acetate = 4:1 as a developing solvent, and t...
Embodiment 2
[0067] Example 2 This example is used to illustrate the preparation method of the recessive malachite green hapten provided by the present invention.
[0068] Add 1.85g of 4-p-bromobenzaldehyde, 2.68g of triphenylphosphine and 0.067g of palladium acetate into a two-neck flask and mix evenly, and then add 40mL of N,N-dimethylformamide (DMF) under the protection of argon as Reaction solvent, then add 3.20mL of methyl 3-butenoate and 2.79mL of triethylamine in sequence, stir (500rpm) at 130°C for reaction, take a small amount every 6 hours and use TLC to judge the progress of the reaction. The reaction ends after about 30h. The reaction solution was poured into 400 mL of ice water, stirred evenly, and then extracted with ethyl acetate 4 times, and the amount of ethyl acetate used each time was 150 mL. After the organic phase was dried by rotary evaporation, it was separated by column using petroleum ether: ethyl acetate = 4:1 as a developing solvent, and the eluting solvent was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com