Myocardial infarction rapid detection kit and preparation method thereof
A kit and rapid technology, applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of delayed treatment, incompleteness, and single data of patients, and achieve economic and social benefits, simple operation, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1. Preparation of MPO-FABP3-cTnI triple quantum dot kit
[0034] 1. Main material
[0035] MPO, FABP3 and cTnI standard products: National Institute of Inspection and Quarantine; MPO, FABP3 and cTnI specific antibodies and antigens: products of Beijing Shengqi Yanghuan Technology Co., Ltd.; quantum dots: products of Wuhan Jiayuan Company; nitrocellulose (NC) membrane: SARTORIUS ( Germany), CN140, product of Sartorius, Germany; bovine serum albumin (BSA), polyethylene glycol PEG20000, hydrolyzed casein: Sigma product; goat anti-mouse IgG antibody: Hangzhou Qitai product; other commonly used reagents are analytical Pure reagents.
[0036] The clinical samples were obtained by the company in relevant hospitals, with a total of 200 serum samples.
[0037] 2. Preparation method
[0038] (1) Quantum dot labeling: Dissolve quantum dot particles with a particle size of 5-10nm in PBS with a pH of 7.40.01M to make a solution with a concentration of 2umol, and add sp...
Embodiment 2
[0043] Embodiment 2. detection method
[0044] (1) Equilibrate the test reagents and samples to room temperature, take out the test paper card, and lay it flat;
[0045] (2) Accurately draw 10 μl of serum, add it to a clean centrifuge tube, dilute it 10 times with sample diluent (PBS or saline), and mix thoroughly;
[0046] (3) Use a pipette gun to draw 50-100ul of the diluted sample and add it to the sample hole, and after standing for 15-20 minutes, use a fluorescence quantitative reader to determine the quantitative result;
[0047] (3) When the instrument judges, after setting the relevant parameters of the instrument, put the test paper card into the chamber for detection, and the instrument will display the quantitative determination result of the sample concentration.
Embodiment 3
[0048] Example 3. Clinical sample detection
[0049] For the detection of 20 clinical value samples, when using the instrument to quantitatively determine the results, the average deviation of the 20 samples within the detection range is less than 15%, the maximum deviation is less than 20%, R2>0.97, and the consistency coefficient>0.88. The test results show that the prepared detection kit has good performance and is suitable for clinical detection.
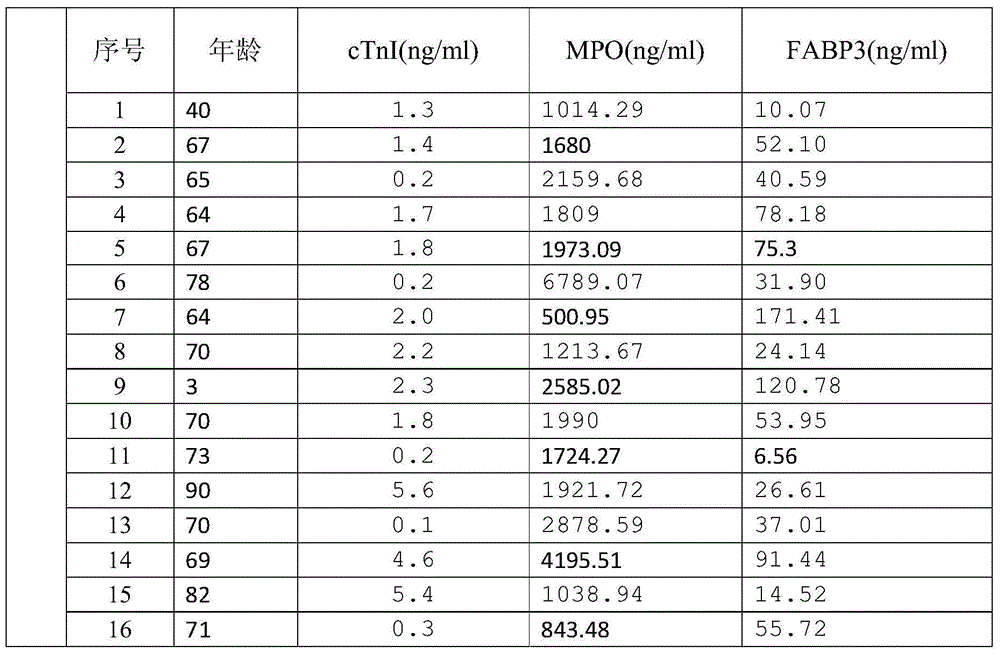
[0050] 16 patients with clinically diagnosed myocardial infarction were tested with the kit of the present invention, and the measured data are shown in Table 1.
[0051] Table 1. Serum detection results of patients with myocardial infarction by MPO-FABP3-cTnI triple kit
[0052]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com