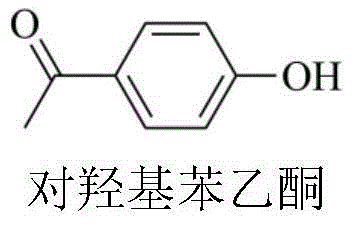
Method for preparing p-hydroxyacetophenone by catalytically oxidizing paraethyl phenol with metalloporphyrin-metal salt composite catalyst
A technology of p-hydroxyacetophenone and composite catalysts, which is applied in the direction of organic compound/hydride/coordination complex catalysts, carbon-based compound preparation, chemical instruments and methods, etc., and can solve the problems of large catalyst consumption, environmental pollution, and three waste discharges To reduce resource consumption and operating costs, shorten response time, and save operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
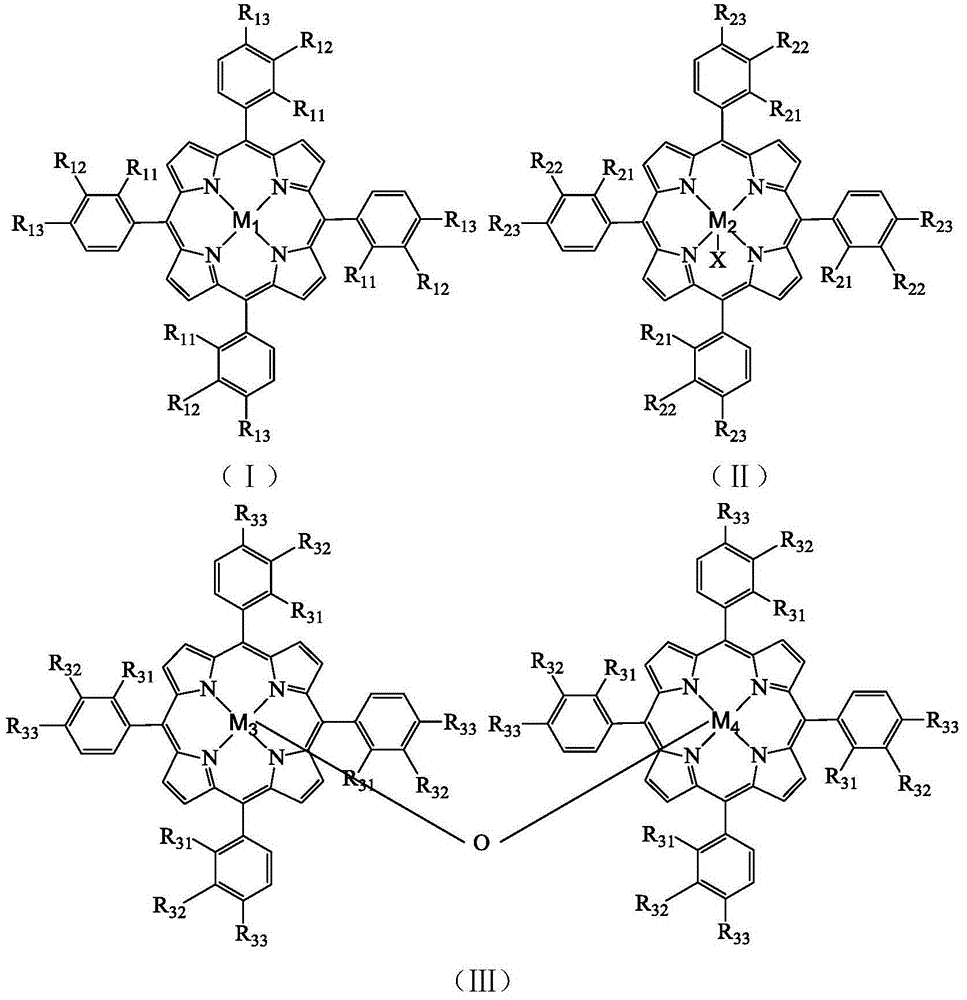
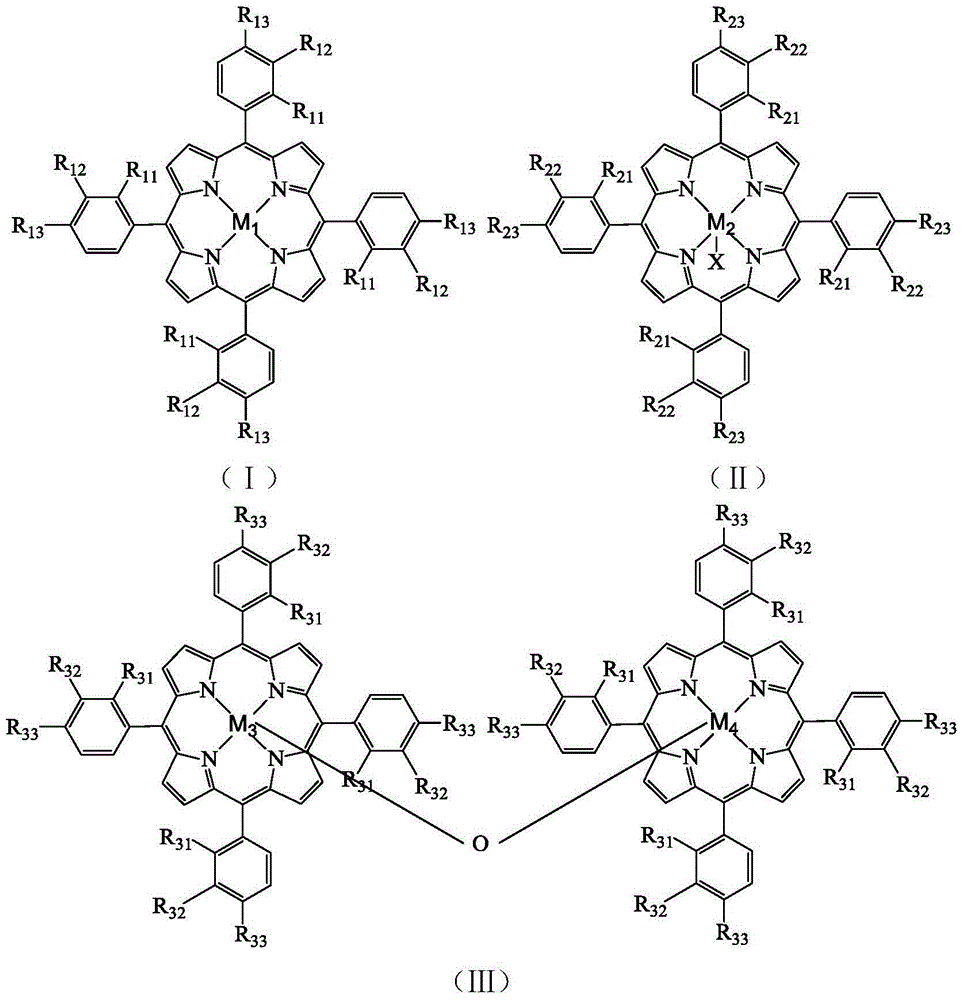
[0030] In a 100mL autoclave, add 12.2g p-ethylphenol successively, 4×10 -3 g tetraphenyl cobalt porphyrin chloride (i.e. R in the general formula (II) 21 for H, R 22 for H, R 23 for H, M 2 is Co, X is Cl), 8g of sodium hydroxide, 30mL of methanol, and oxygen at a pressure of 0.3MPa, reacted at 85°C for 4h. The reacted mixture was analyzed by high-performance liquid chromatography, and the selectivity of 4-hydroxyacetophenone was 31.42%, and the yield was 10.52%.
Embodiment 2
[0032] In a 100mL autoclave, add 12.2g p-ethylphenol successively, 4×10 -3 g tetraphenyliron porphyrin chloride (i.e. R in the general formula (II) 21 for H, R 22 for H, R 23 for H, M 2 Fe, X is Cl), 8g of sodium hydroxide, 30mL of methanol, the pressure of 0.3MPa of oxygen was introduced, and the reaction was carried out at 85°C for 4h. The reaction mixture was analyzed by high-performance liquid chromatography, and the selectivity of 4-hydroxyacetophenone was 16.84%, and the yield was 6.9%.
Embodiment 3
[0034] In a 100mL autoclave, add 12.2g p-ethylphenol successively, 4×10 -3 g tetrakis chloride - (p-methoxyphenyl) manganese porphyrin (i.e. R in the general formula (II) 21 for H, R 22 for H, R 23 for OCH 3 ,M 2 is Mn, X is Cl), 8g of sodium hydroxide, 30mL of methanol, and the oxygen at a pressure of 0.3MPa was introduced to react at 60°C for 4h. The reacted mixture was analyzed by high-performance liquid chromatography, and the selectivity of 4-hydroxyacetophenone was 15.67%, and the yield was 2.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com