Ferulate compound and preparation method thereof
A technology of ferulic acid ester and ferulic acid, applied in the field of medicine, can solve problems such as unsatisfactory effects of finished medicines, and achieve the effect of preventing angioneurotic headaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
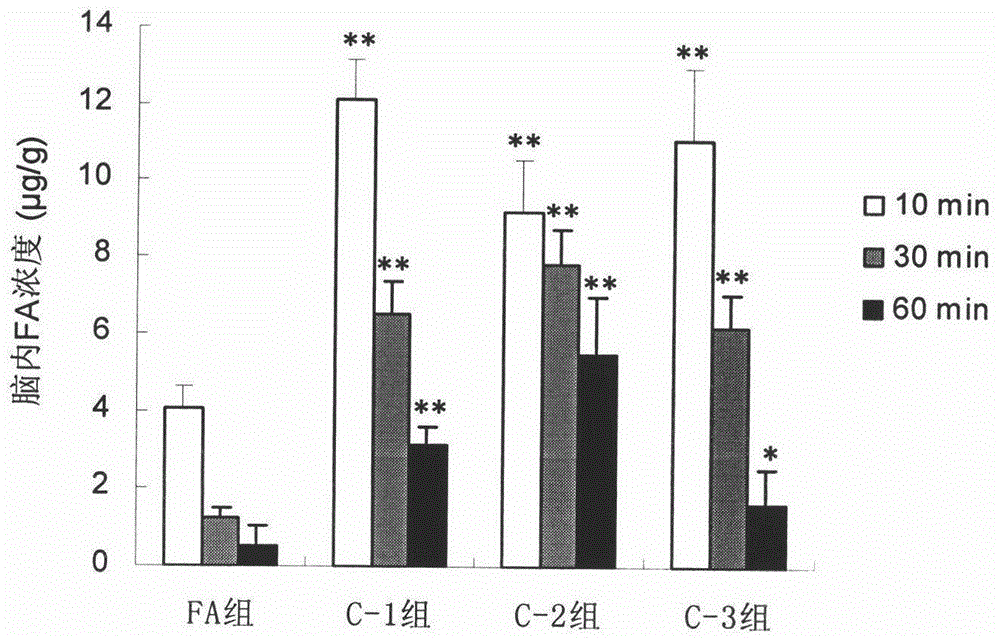
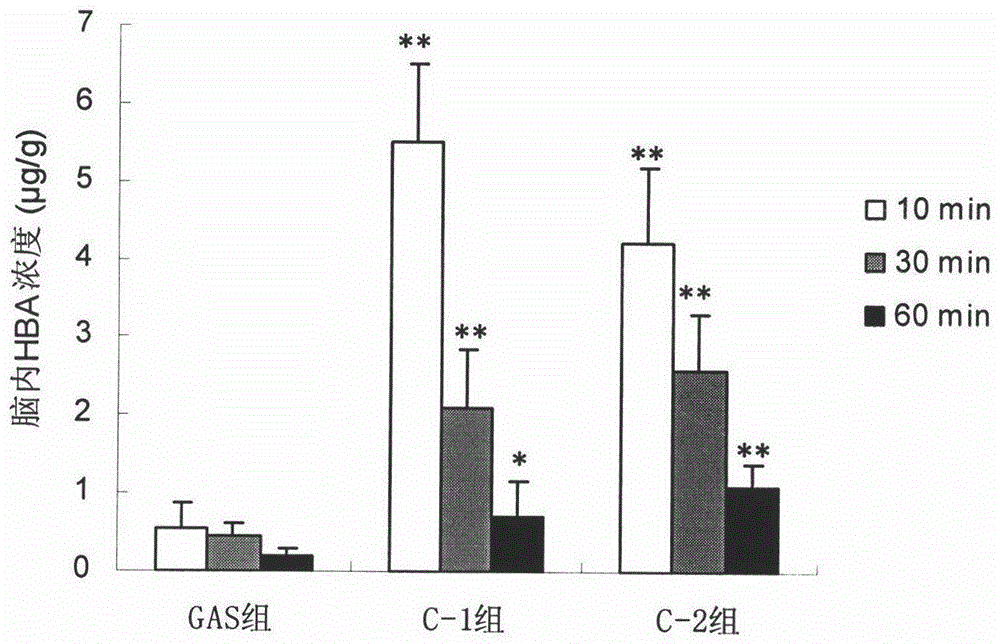
[0052] Example 1 Preparation of Ferulic Acid-4-Hydroxymethylphenol Ester (C-1)
[0053] Add 45 mmol of 4-hydroxybenzyl alcohol, 18 mmol of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, 145 mg of 4-dimethylaminopyridine and CH 2 Cl 2 - THF (1:1, v / v) 100ml, placed in an ice bath and stirred to dissolve. Ferulic acid 15mmol dissolved in CH 2 Cl 2 - 50ml of THF (1:1, v / v) was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to room temperature and the reaction was stirred for 12-24 h (the reaction progress was monitored by TLC, petroleum ether-ethyl acetate=2:1, v / v). After the reaction, the reaction solution was concentrated to a paste, dissolved in ethyl acetate and washed with 1% hydrochloric acid, the organic phase was concentrated and separated on a reversed-phase silica gel column (eluent: 30% to 50% ethanol, v / v) , to obtain ferulic acid-4-hydroxymethylphenol ester (yield 21%).
[0054] 4-hydroxym...
Embodiment 2
[0055] The preparation of embodiment 2 ferulic acid-4-hydroxybenzyl alcohol ester (C-2)
[0056] Add 20mmol of p-hydroxybenzyl alcohol, 200mg of 4-methylbenzenesulfonic acid and 50ml of THF into the reaction flask, stir and dissolve in an ice bath. 15 mmol of dicyclohexylcarbodiimide was dissolved in 15 ml of DMF and added dropwise to the reaction system. Dissolve 10 mmol of ferulic acid in CH 2 Cl 2 After 30ml, it was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to room temperature and the reaction was stirred for 48-72 hours (the reaction progress was monitored by TLC, petroleum ether-ethyl acetate=2:1, v / v). Suction filtration after completion of the reaction, the filtrate was concentrated to a paste, dissolved in ethyl acetate and filtered to remove insoluble matter, the filtrate was washed with 5% hydrochloric acid, the organic phase was concentrated and then purified on a silica gel column (eluent: petroleum e...
Embodiment 3
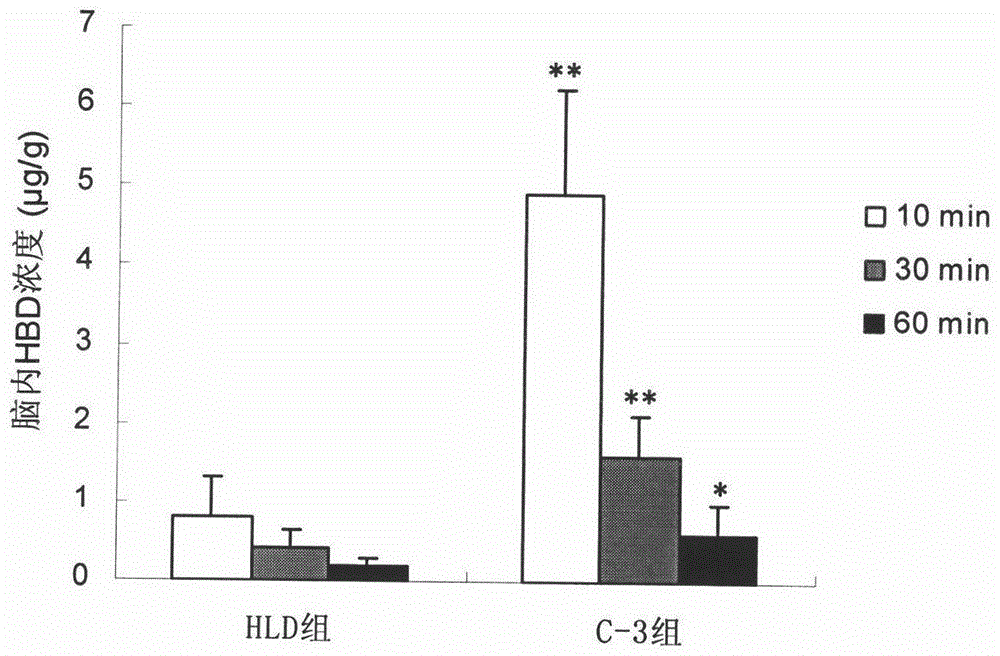
[0058] Example 3 Preparation of Ferulic Acid-4-Formylphenol Ester (C-3)
[0059] Add 60mmol of 4-hydroxybenzaldehyde, 50mmol of dicyclohexylcarbodiimide, 240mg of 4-dimethylaminopyridine and CH 2 Cl 2 - THF (1:1, v / v) 200ml, placed in an ice bath and stirred to dissolve. Ferulic acid 40mmol dissolved in CH 2 Cl 2 - 100ml of THF (1:1, v / v) was slowly added dropwise to the reaction system. After the dropwise addition, the temperature was raised to room temperature and the reaction was stirred for 10-24 h (the reaction progress was monitored by TLC, petroleum ether-ethyl acetate=2:1, v / v). Suction filtration after completion of the reaction, the filtrate was concentrated to a paste, dissolved in ethyl acetate and filtered to remove insoluble matter, the filtrate was washed with 5% hydrochloric acid, the organic phase was concentrated and then purified on a silica gel column (eluent: petroleum ether-ethyl acetate =3:1, v / v) to obtain ferulic acid-4-formylphenol ester (yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com