Immobilized catalytic synthesis method of allyl glycidyl ether molecular sieve
A technology for the synthesis of allyl glycidyl ether and its synthesis method, which is applied in the field of molecular sieve immobilized catalytic synthesis of allyl glycidyl ether, which can solve the problems of difficult recovery of allyl alcohol, high production cost, and environmental pollution, and achieve selectivity High, easy to separate, low corrosion effect of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
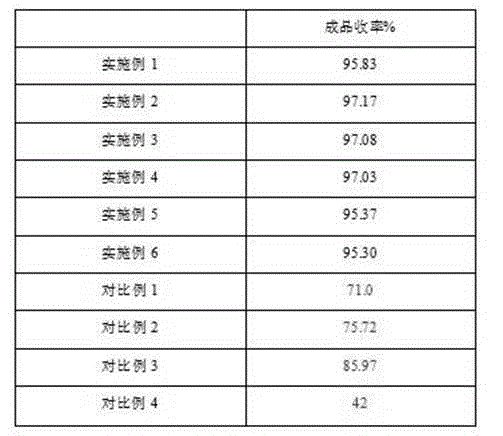
Examples
Embodiment 1
[0027] The preparation method of molecular sieve immobilized concentrated sulfuric acid and trifluoromethanesulfonic acid mixed catalyst: mix 5 parts of concentrated sulfuric acid and 15 parts of trifluoromethanesulfonic acid evenly in advance, add 50 parts in a certain reaction kettle, wash with distilled water and dry in advance 3A molecular sieve, gradually add the mixture of concentrated sulfuric acid and trifluoromethanesulfonic acid dropwise while stirring, then let it stand for adsorption for 12 hours, then dry it at 120°C for 4 hours, after cooling, wash it in absolute ethanol, and put it in 120°C Dry and set aside.
[0028] In the reaction kettle, add 278 parts of allyl alcohol and 6 parts of immobilized catalyst respectively, start stirring, raise the temperature to 45°C, add 185 parts of epichlorohydrin dropwise, control the reaction temperature at 50-55°C, finish adding in 3 hours, and keep warm React for 2 hours, after the reaction is complete, lower the temperatu...
Embodiment 2
[0030] The preparation method of the mixed catalyst of concentrated sulfuric acid and trifluoromethanesulfonic acid immobilized on molecular sieves: 10 parts of concentrated sulfuric acid and 10 parts of trifluoromethanesulfonic acid are uniformly mixed in advance. Add 50 parts of 3A molecular sieves that have been washed and dried with distilled water in a certain reaction kettle, and gradually add the mixture of concentrated sulfuric acid and trifluoromethanesulfonic acid dropwise while stirring, then let it stand for adsorption for 12 hours, and then red at 120°C for 4 hours. , after cooling, wash in absolute ethanol, and dry at 120°C for later use.
[0031] Add 230 parts of allyl alcohol and 16 parts of immobilized catalyst into the reaction kettle, start stirring, raise the temperature to 45°C, add 185 parts of epichlorohydrin dropwise, control the reaction temperature at 50-55°C, complete the addition in 3 hours, and keep warm for the reaction 2h, the reaction is complet...
Embodiment 3
[0033] Add 230 parts of allyl alcohol to the reaction kettle, use the solid-supported catalyst separated in Example 2, start stirring, raise the temperature to 45°C, add 185 parts of epichlorohydrin dropwise, and control the reaction temperature to 50-60°C for 3h After the addition is completed, keep the temperature for 2 hours. After the reaction is completed, lower the temperature, filter out the immobilized catalyst, and recover 110 parts of excess allyl alcohol under reduced pressure; lower the temperature to 30°C, add 5 parts of benzyltriethylammonium chloride, and drop Add 166 parts of 48% liquid caustic soda, control the reaction temperature at 40-45°C, complete the addition in 1 hour, keep the temperature for 2 hours, and complete the reaction. Add 100 parts of water and 100 parts of cyclohexane dissolved salt, let it stand for stratification, drain the brine, add 50 parts of water for washing, and adjust the pH of the water phase to 6-7, drain the lower water phase and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com