Tripeptide wrinkle-reducing compound containing 3,4-dehydro-L-proline residue as well as preparation method and application thereof
A compound and composition technology, applied in the field of tripeptide anti-wrinkle compounds and their preparation, can solve the problems of failing to show wrinkle-reducing effect, failing to show clinical applicability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
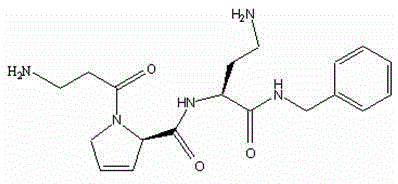
[0059] Example 1: Preparation of H—(β-Ala)—(3,4-Dehydro-Pro)—Dab—NH—Bzl and its acetate.
[0060] 1) Preparation of Fmoc-Dab(Boc)-2-Cl-Trt resin.
[0061] Weigh 8g (8.8mmol) of 2-Cl-Trt resin and 3.88g (8.8mmol) of Fmoc-Dab(Boc)-OH, and add it into a ground-mouthed round bottom flask, then add DIPEA15.7ml (88mmol) and DCM80ml , Shake well, seal and shake on a shaker for 18 hours. Wash the resin with DMF, then add 85ml of DCM, 10ml of methanol and 5ml of DIPEA to block for 20min. After blocking, the resin was washed four times with DMF (60ml) and twice with DCM (60ml). Aspirate the solution. Shrink the resin three times with 60ml, 30ml and 30ml methanol respectively. Vacuum dry. Weighs 9.1g. Store in dry and low temperature. As determined by FMOC absorption method, the amino acid substitution amount is 0.65mmol / gram of resin.
[0062] 2) Preparation of H-Dab(Boc)-2-Cl-Trt resin.
[0063] Weigh 9.1 g of dry Fmoc-Dab(Boc)-2-Cl-Trt resin into a solid-phase reaction synth...
Embodiment 2
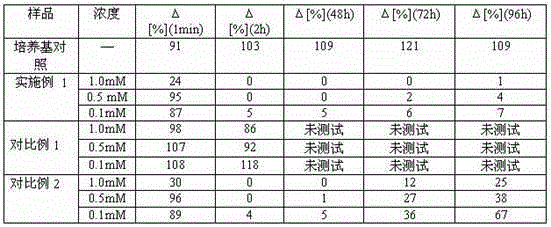
[0084] Example 2 In vitro model testing.
[0085] 1) Materials.
[0086] Normal human muscle cells (myoblasts) at the third passage
[0087] Spinal cord explants of 13-day-old rat embryos with "dorsal root ganglia"
[0088] Medium: 2 / 3MEM and 1 / 3M199, 2mML-glutamine, 50UI / ml penicillin, 50μg / ml streptomycin, 5% fetal bovine serum
[0089] Culture conditions: 37°C, 5% CO 2 .
[0090] 2) Test method.
[0091] To test the efficacy of the compounds of the present invention, a co-culture model of human myocytes and neurons from the spinal cord of rat embryos was established.
[0092] Normal human muscle cells (myoblasts) are cultured in gelatin-coated 24-well plates until a monolayer of myofibrils (from confluent myocytes) forms. Spinal cord explants of 13-day-old rat embryos with dorsal root ganglia were then placed on the myocyte monolayer. After 1 day of co-culture, the first neurite outgrowth from the explant was visible in contact with the myocyte. The first contract...
Embodiment 3
[0099] Example 3 Formulation of the composition.
[0100] 1) Preparation of ointment
[0101] .
[0102] Preparation steps: Heat raw material A (ie, compounds No. 1 to 5) to 70°C. Starting material B (ie, compounds Nos. 6 and 7) was heated to 75°C. Add raw material B to raw material A under stirring condition, cool to 50°C, homogenize and cool to 30°C. Then, raw material component C (ie, compounds No. 8 and 9) and raw material D (ie, compound hydrochloride of Example 1 of the present invention) were added in sequence, and stirred evenly.
[0103] 2) Preparation of gel
[0104] .
[0105] Preparation steps: Dissolve the raw materials numbered 2 to 6 in deionized water in sequence. Then adjust the pH to 6.0 with NaOH, and finally add the compound acetate of Example 1, and stir evenly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com