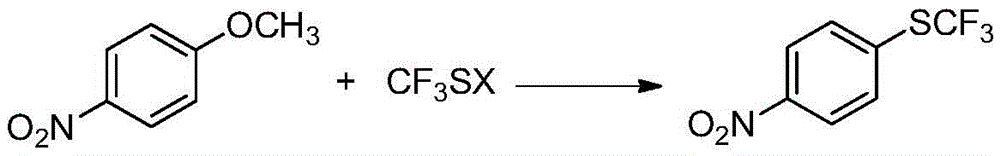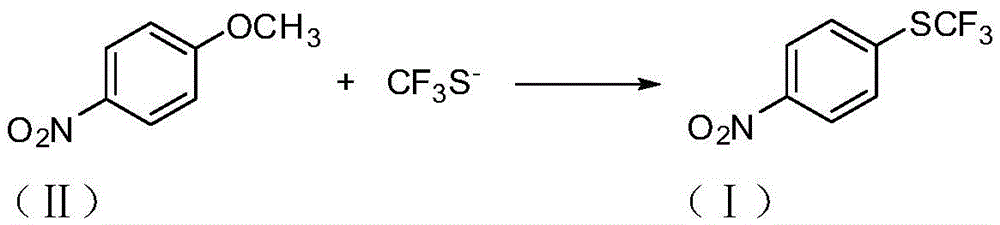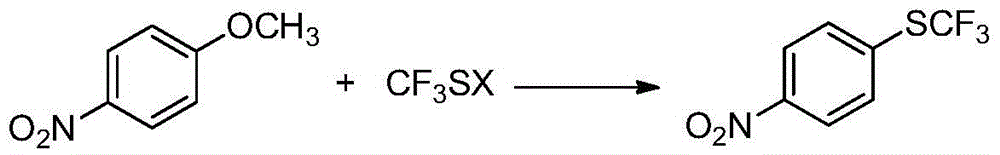A kind of preparation method of 4-trifluoromethylthionitrobenzene
A technology of trifluoromethylthio and trifluoromethylmercaptan, which is applied in the fields of sulfide preparation and organic chemistry, and can solve problems such as high safety production equipment and operation requirements, unsatisfactory overall product yield, and complicated post-processing. , to achieve the effects of extending the continuous production cycle, improving safety and stability, and reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Preparation of 4-trifluoromethylthionitrobenzene (I)
[0023] In the reactor, add p-nitroanisole (II) (153g, 1.0mol), DMF (1800g), stir well and then add sodium trifluoromethanethiolate (148.8g, 1.2mol) in batches. Heat up to 120°C, stir and react for 8 hours. After the reaction is complete, remove the insoluble matter by filtration, then distill off most of the solvent, pour the residue into water, add ethyl acetate (1224g) for extraction, separate the layers, and use anhydrous Sodium sulfate was dried, after filtering to remove the desiccant, the solvent was distilled off, the residue was distilled under reduced pressure, and the fraction at 114-125°C / 2660Pa was collected to obtain 4-trifluoromethylthionitrobenzene (I), 181.6g, The yield is about 81.4%.
[0024] 1HNMR (CDCl 3 , 400MHz) δ: 7.48-7.56 (2H, m), 8.05-8.18 (2H, m). m / z: 224 (M+H)+ Example 2: Preparation of 4-trifluoromethylthionitrobenzene (I)
Embodiment 2
[0024] 1HNMR (CDCl 3 , 400MHz) δ: 7.48-7.56 (2H, m), 8.05-8.18 (2H, m). m / z: 224 (M+H)+ Example 2: Preparation of 4-trifluoromethylthionitrobenzene (I)
[0025] Add p-nitroanisole (II) (153g, 1.0mol) and DMF (1250g) in the reactor, stir well and then add sodium trifluoromethanethiolate (124g, 1.0mol) in batches, and heat up after the addition to 60°C, stirred and reacted for 4 hours, after the reaction was completed, filtered to remove insoluble matter, then distilled off most of the solvent, poured the residue into water, added ethyl acetate (1224g) for extraction, separated liquids, and used anhydrous sulfuric acid for the organic phase Sodium drying, after filtering to remove the desiccant, the solvent was distilled off, the residue was distilled under reduced pressure, and the fraction at 114-125°C / 2660Pa was collected to obtain 4-trifluoromethylthionitrobenzene (I), 153.7g, The rate is about 68.9%.
[0026] 1HNMR (CDCl 3 , 400MHz) δ: 7.48-7.56 (2H, m), 8.05-8.18 (2H, ...
Embodiment 3
[0027] In the reactor, add p-nitroanisole (II) (153g, 1.0mol), DMF (1500g), stir well and then add sodium trifluoromethanethiolate (136.4g, 1.1mol) in batches. Heat up to 90°C, stir and react for 6 hours. After the reaction is complete, remove the insoluble matter by filtration, then distill off most of the solvent, pour the residue into water, add ethyl acetate (1224g) for extraction, separate the layers, and use anhydrous Sodium sulfate was dried, after filtering to remove the desiccant, the solvent was distilled off, the residue was distilled under reduced pressure, and the fraction at 114-125°C / 2660Pa was collected to obtain 4-trifluoromethylthionitrobenzene (I), 172.3g, The yield is about 77.2%.
[0028] 1HNMR (CDCl 3 , 400MHz) δ: 7.48-7.56 (2H, m), 8.05-8.18 (2H, m). m / z: 224(M+H)+Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com