Benzimidazole compounds, synthesis and application of benzimidazole compounds in OLEDs (organic light-emitting diodes)
A technology of benzimidazole and phenylbenzimidazole is applied in the field of organic electroluminescent materials, which can solve the problems of complicated synthesis, increase production cost and cycle, etc., and achieve the effect of improving device performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
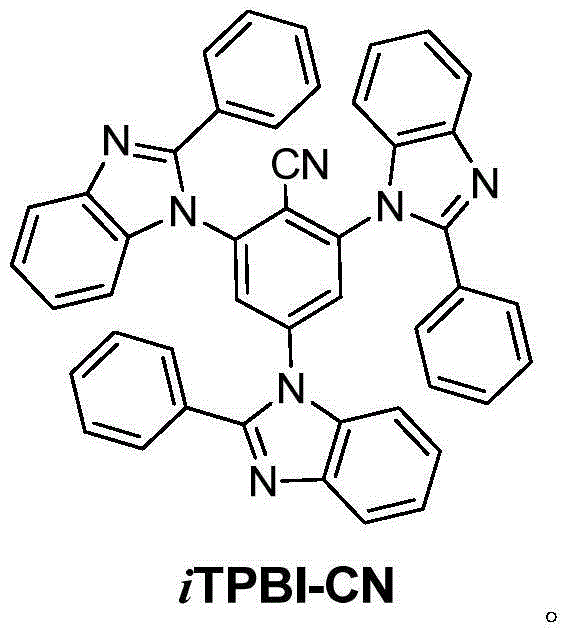
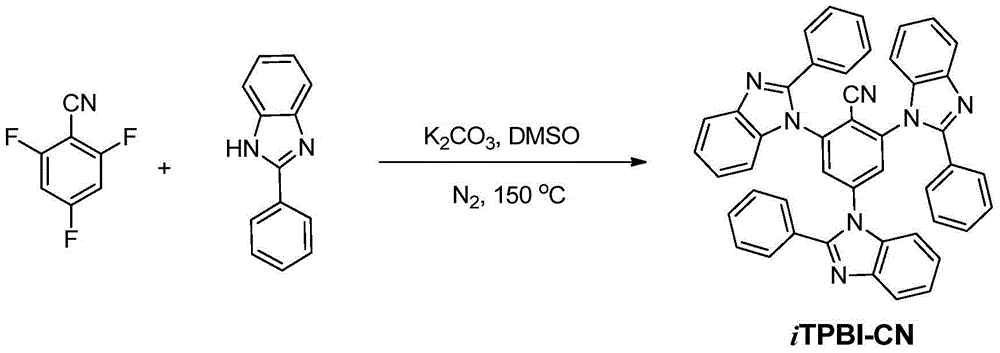
[0016] Embodiment (1): the synthesis of iTPBI-CN
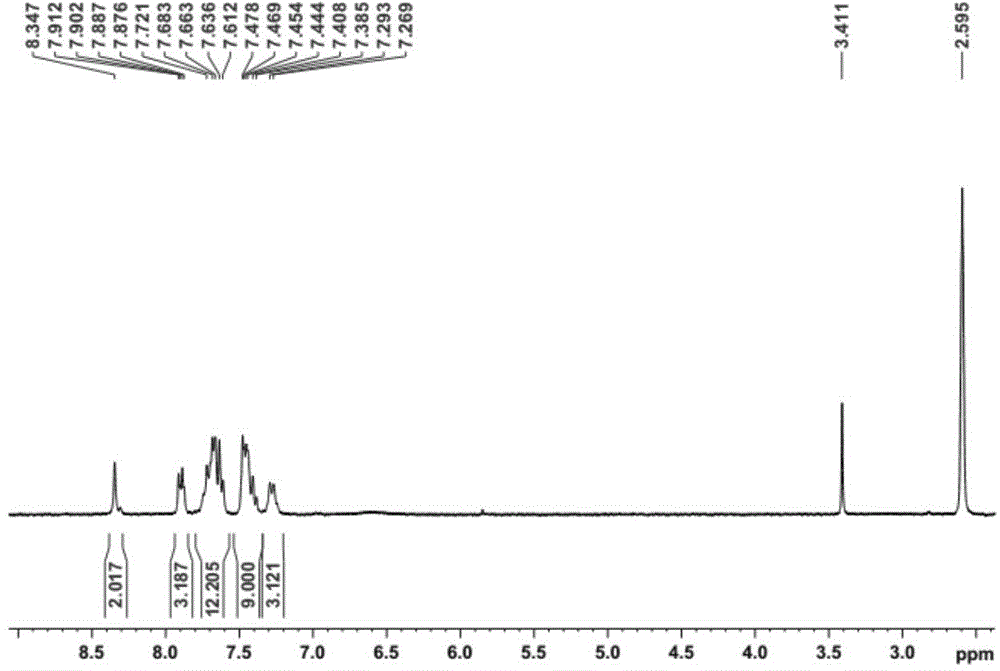
[0017] 2,4,6-Trifluorobenzonitrile (0.25g, 1.6mmol), potassium carbonate (0.99g, 7.2mmol), 2-phenylbenzimidazole (1.02g, 5.2mmol), DMSO 8ml, heated to reflux at 150°C 12h. Cooled to room temperature and poured into 200ml of water to precipitate a large amount of solid, stirred for 0.5h, filtered with suction to obtain a white solid, and purified by column chromatography to obtain 0.95g of a white solid, with a yield of 88%. Its hydrogen spectrum is shown in figure 1 . 1 H NMR (300MHz, (CD 3 ) 2 SO): δppm 8.34(s,2H),7.91-7.88(m,3H),7.72-7.61(m,12H),7.48-7.38(m,9H),7.29-7.27(d,3H,J=6Hz) ; 13 C NMR (75MHz, (CD 3 ) 2 SO,): δppm 155.2, 155.1, 145.8, 145.7, 145.6, 144.3, 138.8, 133.3, 133.1, 132.9, 132.6, 132.3, 132.2, 132.0, 126.9, 126.6, 122.8, 122.7, 115.2, 113.114. , 113.3; mass spectrum (m / z): 679.637[M+]; elemental analysis C46H29N7: calculated C 81.28, H 4.30, N 14.42; experimentally measured C 81.35, H 4.09, N 14.58...
Embodiment (2
[0020] Embodiment (2): solution method prepares organic electroluminescent device
[0021] The organic electroluminescent device includes glass, a conductive glass substrate layer attached to the glass, a hole injection layer bonded to the conductive glass substrate layer, a light-emitting layer bonded to the hole injection layer, and an electron transport layer bonded to the light-emitting layer. layer, and a cathode bonded to the electron transport layer, wherein the electron transport layer is prepared by using the iTPBI-CN described in the present invention and the TPBI described in the prior art respectively.
[0022] The ITO glass substrate was ultrasonically treated with detergent, organic solvent mixed with ethanol and acetone, and deionized water, and then dried in a drying oven at 120 ° C for more than 1 h. Take out the ITO substrate, after UV treatment for 4min, spin 40nm thick PEDOT:PSS, and anneal at 120°C for 15min. Spin a light-emitting layer with a thickness o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com