Barbituric acid chiral cyclohexane spiro compound and its preparation method and use
A technology of spiro compound and barbituric acid, which is applied in the field of barbituric chiral cyclohexane spiro compound and its preparation and application, can solve the problem of unseen barbituric chiral cyclohexane spiro compound. Compound preparation method Antibacterial drugs, no barbituric acid chiral cyclohexane spiro compounds and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] Using propionaldehyde (0.4mmol) and compound 2-a (0.3mmol, commercially available, CAS No. 102-96-5) as raw materials, add (2R)-2-[diphenyl[trimethylsilyloxy]methanol Base]-pyrrolidine as catalyst (0.03mmol), glacial acetic acid (0.04mmol), acetonitrile (1ml) as solvent, stirred at room temperature for 3-4 hours to obtain compound 3-a.
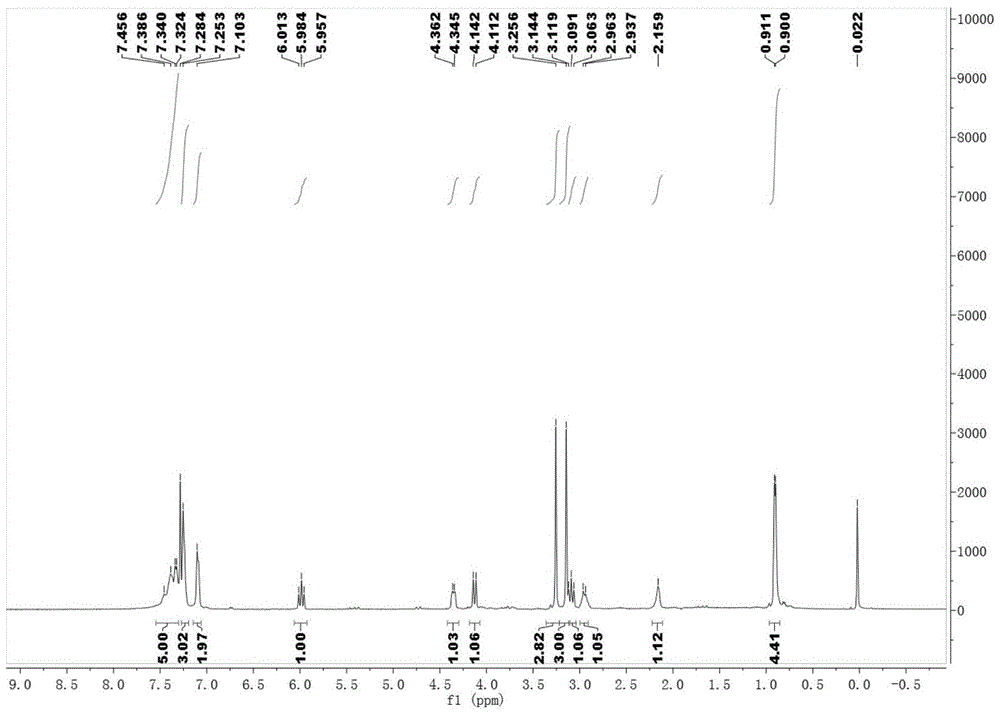
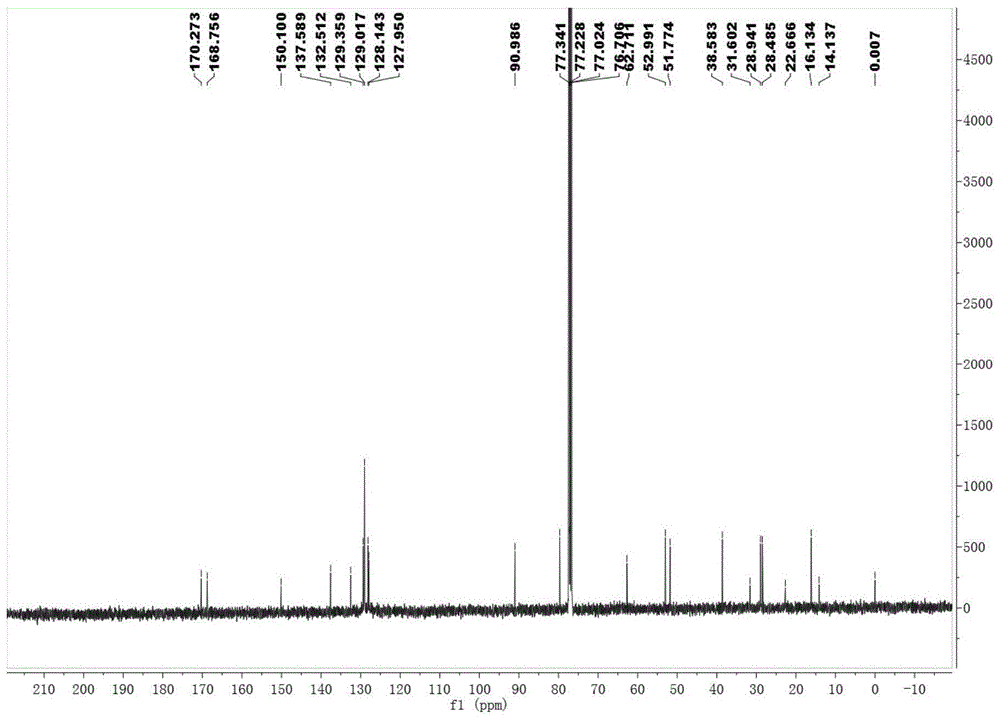
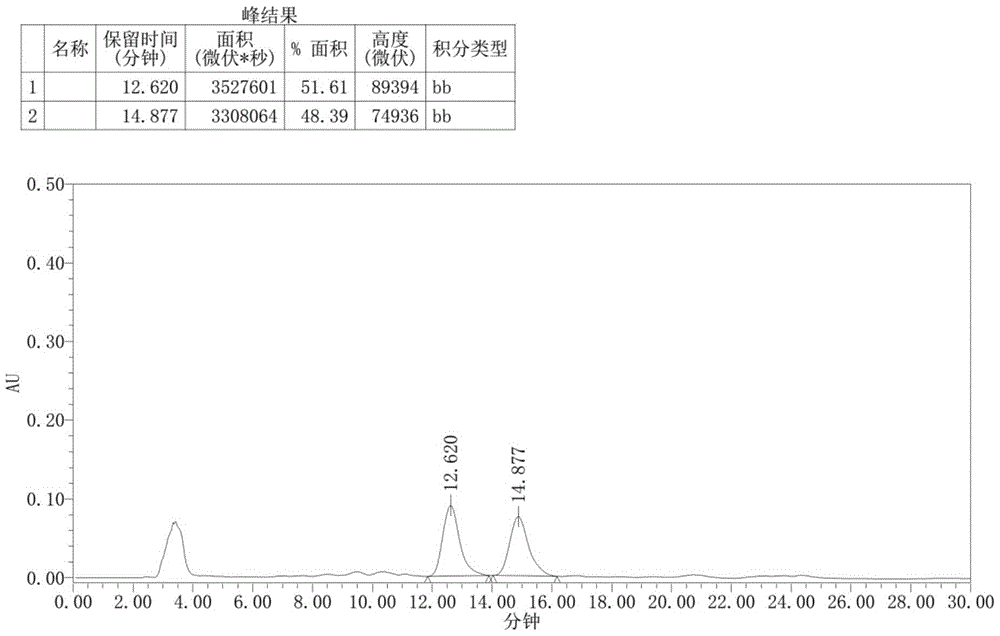
[0082] After the reaction was completed, compound 4-a (0.2mmol), potassium carbonate (0.1mmol dissolved in 1.0mL water) and TBAB (0.01mmol; tetrabutylammonium bromide) were directly added in one pot, and the reaction was stirred at 60°C. The reaction was completely monitored by TLC (thin layer chromatography) to obtain a reaction solution; the reaction solution was spin-dried to solvent, and separated and purified by silica gel column chromatography to obtain 55.9 mg of the compound shown in Formula I-a with a yield of 62%, 94% ee , 94:6dr( 1 H NMR analysis).
[0083] The detection data of the compound shown in formula Ⅰ-a...
Embodiment 2
[0092]
[0093] Using propionaldehyde (0.4mmol) and compound 2-b (0.3mmol, commercially available, CAS No. 102-96-5) as raw materials, add (2R)-2-[diphenyl[trimethylsilyloxy]methanol Base]-pyrrolidine as catalyst (0.03mmol), glacial acetic acid (0.04mmol), acetonitrile (1ml) as solvent, stirred at room temperature for 3 to 4 hours to obtain compound 3-b.
[0094] After the reaction was completed, compound 4-b (0.2mmol), potassium carbonate (0.1mmol dissolved in 1.0mL water) and TBAB (0.01mmol; tetrabutylammonium bromide) were directly added in one pot, and the reaction was stirred at 60°C. The reaction was monitored by TLC (thin layer chromatography) to obtain a reaction solution; the reaction solution was spin-dried to solvent, and separated and purified by silica gel column chromatography to obtain 45.1 mg of the compound shown in formula I-b with a yield of 51%, 98% ee , 85:15dr( 1 H NMR analysis).
[0095] The detection data of the compound shown in formula Ⅰ-b is as ...
Embodiment 3
[0104]
[0105] Using propionaldehyde (0.4mmol) and compound 2-c (0.3mmol, commercially available, CAS No. 102-96-5) as raw materials, add (2R)-2-[diphenyl[trimethylsilyloxy]methanol Base]-pyrrolidine as catalyst (0.03mmol), glacial acetic acid (0.04mmol), acetonitrile (1ml) as solvent, stirred at room temperature for 3 to 4 hours to obtain compound 3-c.
[0106] After the reaction was completed, compound 4-c (0.2mmol), potassium carbonate (0.1mmol dissolved in 1.0mL water) and TBAB (0.01mmol; tetrabutylammonium bromide) were directly added in one pot, and the reaction was stirred at 60°C. The reaction was monitored by TLC (thin-layer chromatography) to obtain a reaction solution; the reaction solution was spin-dried to solvent, and separated and purified by silica gel column chromatography to obtain 52.9 mg of the compound shown in formula I-c, with a yield of 68%, 98% ee , 91:9dr( 1 H NMR analysis).
[0107] The detection data of the compound shown in formula Ⅰ-c is as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com