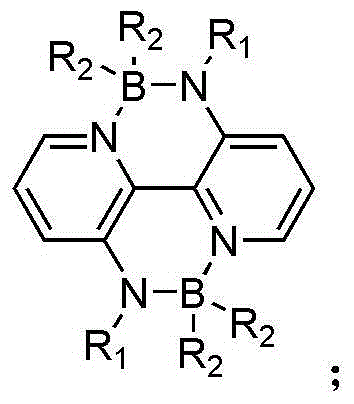
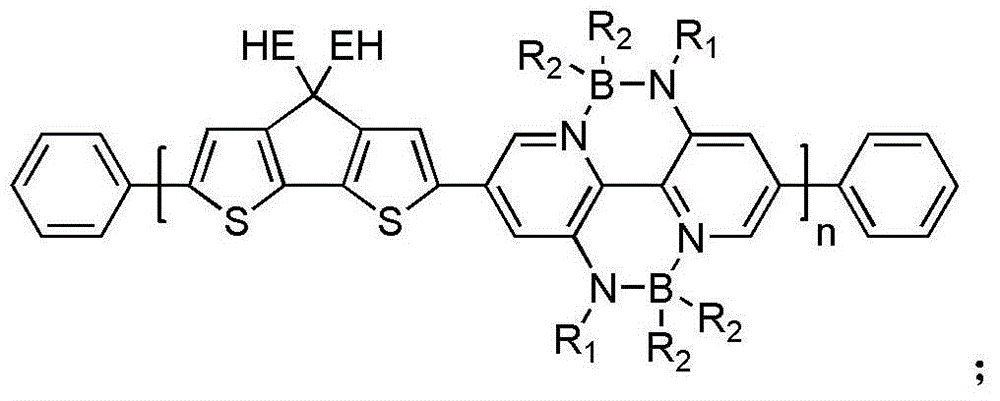
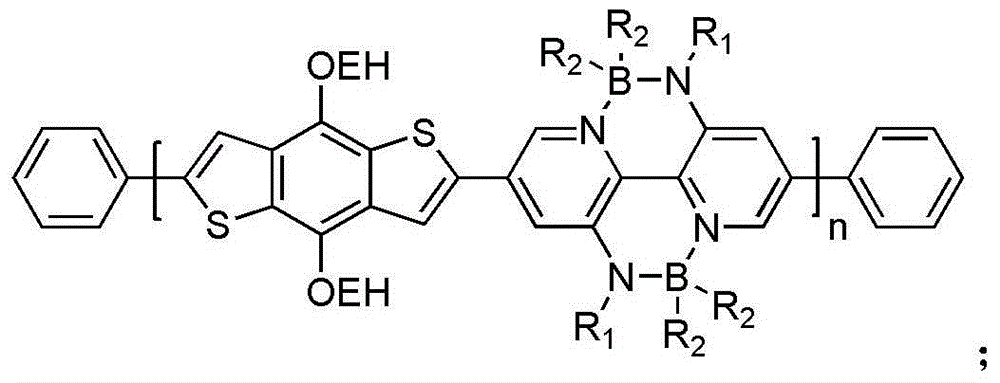
Diboron nitride bridge-linked bipyridine and organic/ high polymer material prepared from diboron nitride bridge-linked bipyridine
A technology of bipyridine and boron nitrogen, which is applied in the field of organic/polymer solar cells, can solve the problems of sensitivity, instability of three-coordinated boron structure, and influence on material properties, and achieve good application prospects and strong π-electron delocalization , the effect of improving the migration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation example of double boron nitrogen bridged bipyridine is as follows:
[0030] Synthesis of Compound 2 (R 1 =-C 4 h 9 ):
[0031] Under the protection of argon, dissolve 2 g of compound 1 in tetrahydrofuran, and cool to -78 ° C, slowly add 2.2 times the equivalent of n-butyllithium dropwise, and then add 3 times the equivalent of Br-C after maintaining this temperature for 2 hours. 4 h 9 , gradually warming up to 65°C and refluxing for 24 hours. Return to room temperature, evaporate the solvent to dryness, and purify by column chromatography (dichloromethane: n-hexane mobile phase) to obtain 3.5g of compound 2. 1H-NMR: (400MHz, CDCl 3 ,25℃);δ9.53(s,1H),7.85(d,J=4.0Hz,1H),7.09(m,J=8.4,4.4Hz,1H),7.01(d,J=8.3Hz,1H ), 3.18 (d, J = 12.1, 6.9 Hz, 2H), 1.75–1.68 (m, 2H), 1.52–1.45 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H). 13C NMR (101MHz, CDCl 3 ,25°C): δ145.53, 140.29, 132.37, 123.05, 117.30, 42.70, 31.37, 20.67, 14.08. The synthetic route is as follows:
[00...
Embodiment 1
[0034] Synthesis of double boron nitrogen bridged bipyridine compound 5a (R 1 =-C 4 h 9 ):
[0035] Under the protection of argon, dissolve 1.5g of compound 2 in dry dichloromethane, slowly add 4 times the equivalent of boron trifluoride ether solution and a certain amount of triethylamine, reflux at 50°C for 2 hours, cool to room temperature, and distill off the solvent , purified by column chromatography (dichloromethane: n-hexane mobile phase) to obtain 1.7 g of compound 5a. 1H NMR: (400MHz, CDCl 3 ,25℃):δ8.16(d,J=4.8Hz,1H),7.57(dt,J=16.1,6.6Hz,2H),3.67–3.54(m,2H),1.70–1.62(m,2H) , 1.49–1.39 (m, 2H), 0.98 (t, J=7.2Hz, 3H). 13C NMR (101MHz, CDCl 3 ,25°C): δ143.58, 127.49, 126.46, 125.95, 122.47, 43.70, 29.98, 20.55, 14.02. Its synthetic route is as follows:
[0036]
Embodiment 2
[0038] Synthesis of double boron nitrogen bridged bipyridine compound 5b (R 1 =-C 4 h 9 ):
[0039] Under the protection of argon, dissolve 1.5 g of compound 2 in dry dichloromethane, slowly add 4 times the equivalent of triphenylboron and a certain amount of triethylamine, reflux at 50 ° C for 2 hours, cool to room temperature, distill off the solvent, and Purification by chromatographic separation (dichloromethane: n-hexane mobile phase) gave 1.8 g of compound 5b. Its synthetic route is as follows:
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com