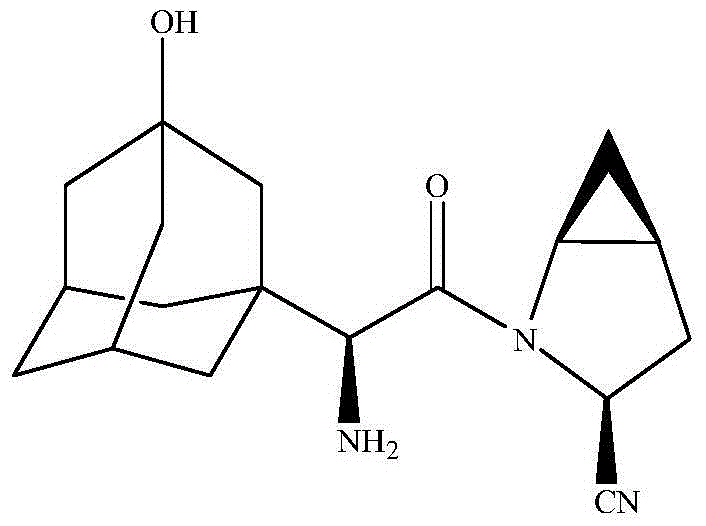
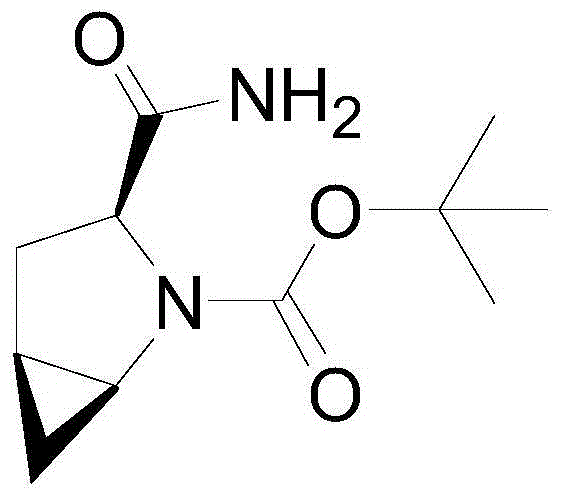
Preparation method of saxagliptin intermediate
An intermediate, azabicyclic technology, applied in the field of medicinal chemistry, can solve the problems of high cost and low yield, and achieve the effect of simple operation, high selectivity and large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
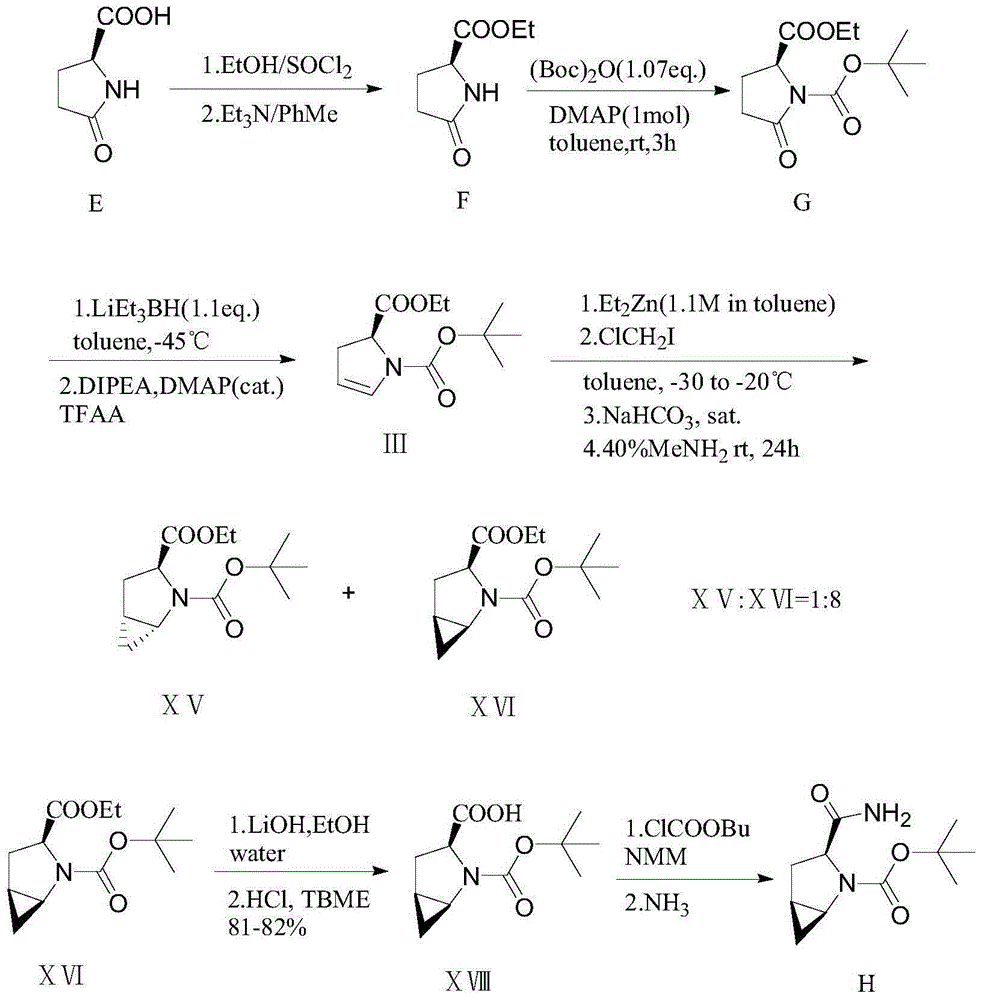
Embodiment 1
[0036] Preparation of ethyl L-pyroglutamate (hereinafter referred to as compound 2)
[0037] In a dry 500ml four-necked reaction flask, N 2 Under protection, add L-pyroglutamic acid (25.9g, 0.20mol) and dissolve it in 250ml of absolute ethanol, keep the temperature of the system at -5°C, and start adding thionyl chloride (SOCl 2 ) (26.2g, 0.22mol), added dropwise for 2h, stirred for 0.5h after the dropwise addition, then raised the temperature to 22°C, stirred for 2 hours, concentrated the solvent under reduced pressure at a water bath temperature of 45°C, and obtained a colorless oil. Then add 100ml of toluene and stir evenly, then concentrate the solvent under reduced pressure at a water bath temperature of 45°C to obtain a colorless oily substance, then add 200ml of toluene to maintain the temperature at 10°C, then add 200ml of triethylamine (TEA) to adjust the pH of the system to 8, then Stir for 0.5h, filter, and wash the filter cake with toluene. The filtrate was conce...
Embodiment 2
[0041] Preparation of Boc-L-ethyl pyroglutamate (hereinafter referred to as compound 3)
[0042] In a dry 500ml four-necked reaction flask, add compound 2 (28.3g, 0.18mol) and dissolve it in 250ml of toluene, add dimethylaminopyridine (DMAP) (11.2g, 0.09mol) and stir, maintain at 14°C and add di Di-tert-butyl carbonate ((Boc) 2 O) (58.9g, 0.27mol) toluene solution system, add dropwise for 1h, keep warm at 25°C after dropwise addition, stir for 3h, then wash once with 10ml4.5% sodium bicarbonate solution, and wash twice with water, Carefully separate the water layer, concentrate the organic layer at 50°C under reduced pressure to obtain a yellow oil, then add 100ml of toluene and stir evenly, and keep the temperature at 50°C, start adding 100ml of n-heptane dropwise, and keep the temperature at 25°C after the dropwise addition After stirring for 2 hours, the system was cooled to 5°C, stirred for 30 minutes, filtered, the filter cake was slurried with n-heptane, sucked dry, and...
Embodiment 3
[0046] Preparation of (S)-1-N-tert-butoxycarbonyl-2-hydroxyl-2-pyrrolecarboxylic acid ethyl ester (hereinafter referred to as compound 4)
[0047] In a dry 250ml three-neck reaction flask, N 2 Add compound 3 (30.9g, 0.12mol) under protection and dissolve it in 180ml tetrahydrofuran (THF), and add a toluene solution of diisobutylaluminum hydride (DIBAL-H) dropwise at a temperature of -65°C. After the dropwise addition is completed, Stir for 1.5h, concentrate under reduced pressure to remove the solvent to obtain a colorless oily substance, then add 100ml of toluene, adjust the pH to 8 with 200ml of triethylamine (TEA), concentrate under reduced pressure, and recrystallize with n-heptane to obtain a white solid, namely compound 4 (23.8 g, 91.8%).
[0048] (S)-1-N-tert-butoxycarbonyl-2-hydroxyl-2-pyrrolecarboxylate ethyl NMR spectrum data are as follows:
[0049] 1 H NMR (400MHz, CDCl 3 ): δ=6.75-6.40(m,1H), 4.98(d,J=19.1Hz,1H), 4.35-4.08(m,2H), 3.19-2.94(m,2H), 2.77-2.54(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com