Pyrazole-containing bishydrazide compound and preparation method and application thereof
A technology of pyrazole bishydrazide and compound, which is applied to pyrazole bishydrazide-containing compounds and the fields of preparation and application thereof, and can solve the problems of few bishydrazide compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 1-(2-Ethyl-phenyl)-5-methyl-1 H- Preparation of pyrazole-4-carboxylic acid chloride
[0022] Add 0.46g (0.002mol) 1-(2-ethyl-phenyl)-5-methyl-1 to a 50mL one-necked flask H- Pyrazole-4-carboxylic acid and 1.428 g (0.012 mol) SOCl 2 , react at room temperature for 1 h, and then reflux for 2 h. After the reaction, remove excess thionyl chloride to obtain a light yellow transparent liquid, which is sealed and stored for future use.
Embodiment 2
[0023] Example 2 Preparation of the target product
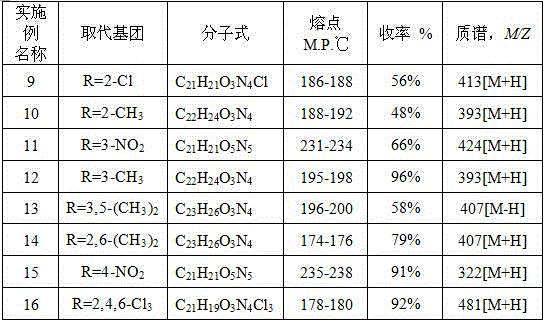
[0024] Add 4-methylphenoxyacetylhydrazide (0.0018 mol), (Et) 3 N, ethyl acetate (10 mL), and then Example 1. Add the mixed solution of acid chloride and ethyl acetate dropwise into the reaction flask, stir overnight at room temperature, solids are precipitated, and monitor by pointing a plate. After the raw materials are completely reacted, suction filter and wash to obtain a light-colored solid powder, namely the product. Melting point 241-243 o C, yield 63%.
[0025] of the compound 1 H NMR and ESI-MS data are as follows:
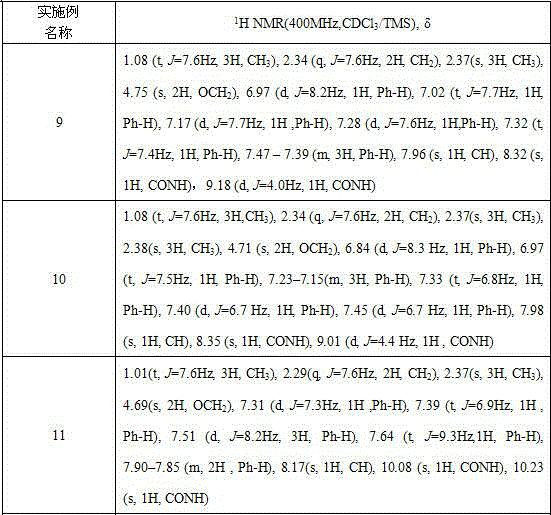
[0026] 1 H NMR (CDCl 3 , d / ppm)δ 1.08 (t, J =7.6Hz, 3H ,CH 3 ), 2.31(s, 3H, CH 3 ), 2.34 (q, J =7.6Hz, 2H, CH 2 ), 2.37(s, 3H, CH 3 ), 4.66 (s, 2H, OCH 2 ), 6.88 (d, J =9.6Hz, 2H, Ph-H), 7.13 (d, J =8.3Hz, 2H, Ph-H), 7.17 (d, J =7.7Hz, 1H, Ph-H), 7.32 (t, J =8.2Hz, 1H, Ph-H), 7.40 (d, J =6.9Hz, 1H, CONH), 7.46 (t, J =7.0Hz, 1H, Ph-H), 7.95 (s, 1H, CH), 8.32 (s, 1H, Ph-H), 9.01 (d, ...
Embodiment 3
[0027] Example 3 Preparation of the target product
[0028] Add 2-nitrophenoxyacetylhydrazide (0.0018 mol), (Et) 3 N. Ethyl acetate (10 mL), then drop the mixed solution of acid chloride and ethyl acetate into the reaction flask, stir at room temperature overnight, there is solid precipitation, spot plate monitoring, after the raw materials are completely reacted, suction filtration, washing, to obtain Light yellow solid powder, the product, melting point 179-182 o C, yield 91%.
[0029] of the compound 1 H NMR and ESI-MS data are as follows:
[0030] δ 1.08 (t, J =7.6Hz, 3H,CH 3 ), 2.35(q, J =7.6Hz, 2H, CH 2 ), 2.31(s, 3H, CH 3 ), 4.85(s, 2H, OCH 2 ), 7.10(d, J =7.8Hz, 1H, Ph-H), 7.19(t, J =8.0Hz, 2H, Ph-H), 7.32 (t, J =7.6Hz, 1H, Ph-H), 7.40 (d,J =7.8Hz, 1H,Ph-H ), 7.46 (t, J =7.5Hz, 1H, Ph-H), 7.68 – 7.61 (m, 1H, Ph-H), 7.95 (s, 1H, CH), 8.06 (s, 1H, Ph-H), 8.08 (s, 1H, CONH), 9.34 (d , J = 4.0 Hz, 1H, CONH). ESI-MS: 424[M+H].
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com