A kind of industrial manufacturing method of glycerophosphocholine
A technology of glycerophosphocholine and industrial manufacturing, which is applied in the direction of phosphorus organic compounds, can solve the problems of high cost, difficult purification, complex reaction, etc., and achieve the effect of avoiding by-product problems and purification problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
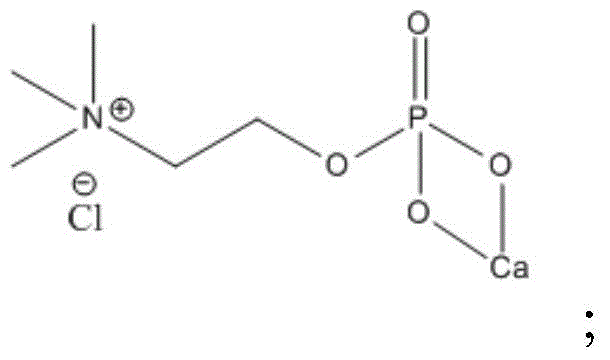
[0046] Add phosphoryl choline calcium salt (generally tetrahydrate) 330g and 300ml water in reactor, stir until dissolving clear, drip the concentration that is made of anhydrous sodium carbonate 106g and water be the sodium carbonate solution of 50%, Add dropwise at a rate of 10ml / min. After the drop is complete, stir at room temperature for 1 hour. According to the different solubility of sodium chloride, separate out the sodium chloride in the reaction system by salting out and filter.
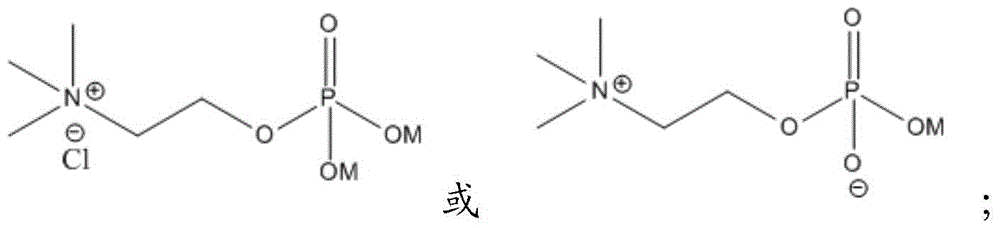
[0047] The filtrate was passed through a sulfonic acid-type ion column, and eluted with water several times until no product was found in HPLC.
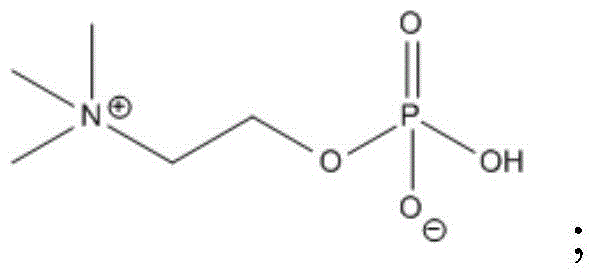
[0048] The solvent was distilled under reduced pressure, concentrated to a volume of 300ml, 66g of (R)-chiral glycidol was added, stirred and refluxed for 2-6 hours, HPLC showed that there was no raw material, the water was evaporated under reduced pressure, and recrystallized in methanol to obtain 289g product. (HPLC>99.7%)
Embodiment 2
[0050] Add phosphorylcholine calcium salt (generally tetrahydrate) 330g and 200ml water in the reaction kettle, stir until dissolving clear, add dropwise the potassium carbonate solution that the concentration that is made of potassium carbonate 138g and water is 35%, with 5ml Add dropwise at a speed of 1 / min, after the drop is completed, stir at room temperature for 0.5 hour, pass through a silica gel column to remove inorganic salt insolubles such as potassium chloride.
[0051] The filtrate was passed through a sulfonic acid-type ion column, eluted with methanol several times until there was no product in HPLC, and evaporated under reduced pressure until there was no distillate.
[0052] Add 300ml of 1,2-dimethoxyethane to dissolve the above substances, stir and drop 66g of (R)-chiral glycidol, and reflux for 8 hours. HPLC shows that there is no raw material, and the water is evaporated under reduced pressure to obtain 291g of product. (HPLC>99.5%)
Embodiment 3
[0054] Add 330g of phosphorylcholine calcium salt (generally tetrahydrate) and 500ml of water into the reaction kettle, stir until it dissolves, add dropwise a 65% sodium oxalate solution made of 133g of sodium oxalate and water, and add 2ml Add dropwise at a speed of 1 / min, after the drop is complete, stir at room temperature until no new precipitation occurs. According to the different solubility of sodium chloride, the sodium chloride in the reaction system is precipitated by salting out and then filtered.
[0055] The filtrate was passed through a sulfonic acid-type ion column, and eluted with 1,2-dimethoxyethane several times until no product was found in HPLC.
[0056] Concentrate to 400ml, stir and add 66g of (R)-chiral glycidol dropwise, and reflux for 7 hours. HPLC shows that there is no raw material, and the water is evaporated under reduced pressure to obtain 295g of product. (HPLC>99.5%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com