Method for preparing optical pure 1-(1-naphthyl)ethylamine through resolution with immobilized enzyme process
An immobilized enzyme and naphthyl technology, applied in fermentation and other fields, can solve the problems of poor resistance to organic solvents and low reusability of immobilized enzymes, and achieve excellent splitting effect and high reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1)
[0024] This example is the preparation method of cross-linked Candida antarctica lipase B aggregates (hereinafter referred to as cross-linked enzyme aggregates):
[0025] 15 mg of Candida antarctica lipase B (hereinafter abbreviated as CLAB) was added to 5 mL of water and dissolved completely to obtain an enzyme liquid.
[0026] Then add 25mL of saturated ammonium sulfate solution to precipitate the enzyme protein from the enzyme solution.
[0027] Then add 0.15mL of 25wt% glutaraldehyde aqueous solution, stir and crosslink for 2h.
[0028] Finally, 15 mg of cross-linked enzyme aggregates were obtained by dialysis and freeze-drying.
example 2~ example 5)
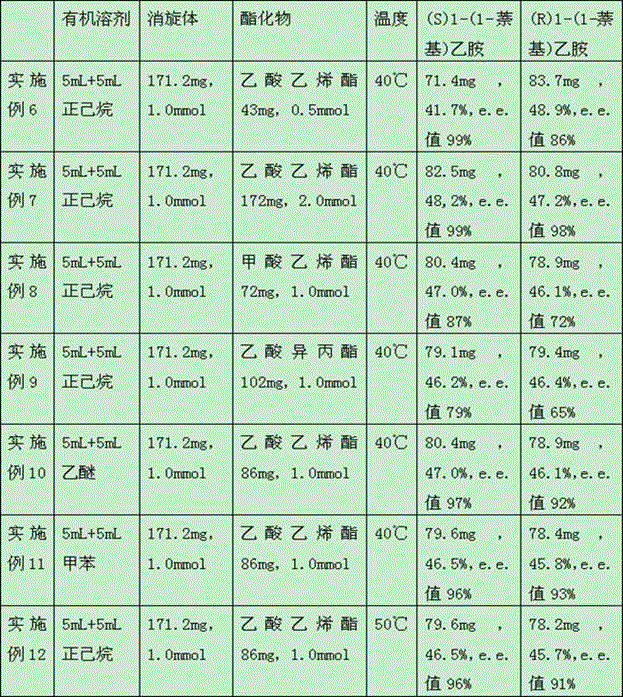
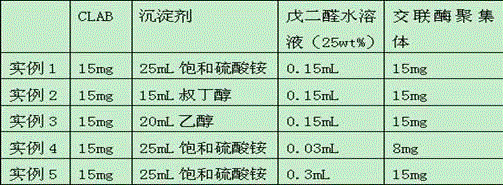
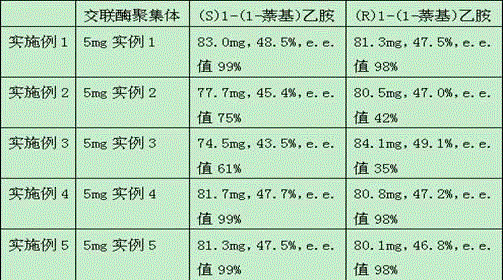
[0030] The method of each example is basically the same as Example 1, see the difference Table 1 .
[0031] Table 1
[0032]
Embodiment 1)
[0034] The method for preparing optically pure 1-(1-naphthyl)ethylamine by immobilized enzymatic resolution in this embodiment has the following steps:
[0035] ①In 5 mL of n-hexane solvent, add 5 mg of the cross-linked enzyme aggregate prepared in Example 1 and 171.2 mg of 1-(1-naphthyl)ethylamine racemate (1.0 mmol, hereinafter referred to as racemate) , the temperature of the reaction system was raised to 40°C, and 5 mL of n-hexane solvent containing 86 mg of vinyl acetate (1.0 mmol) was added to the reaction system within 1.5 h. A mixture of 1-(1-naphthyl)ethylamine ester and (S)1-(1-naphthyl)ethylamine.
[0036] ② After the cross-linked enzyme aggregates were separated by filtration, they were distilled under reduced pressure to obtain light yellow oil.
[0037] Using petroleum ether and isopropanol (1:1) as the mobile phase, the oil was separated by column chromatography to obtain (R)1-(1-naphthyl)ethylamino ester and 83.0 mg of (S)1-( 1-Naphthyl)ethylamine, yield 48.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com