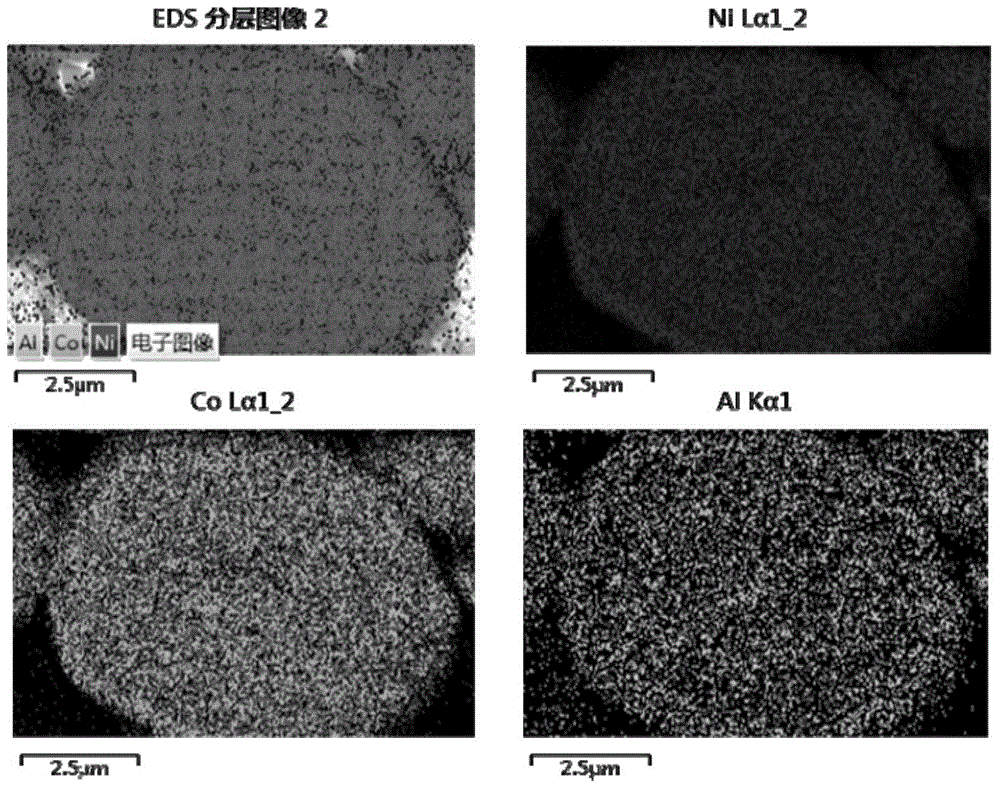
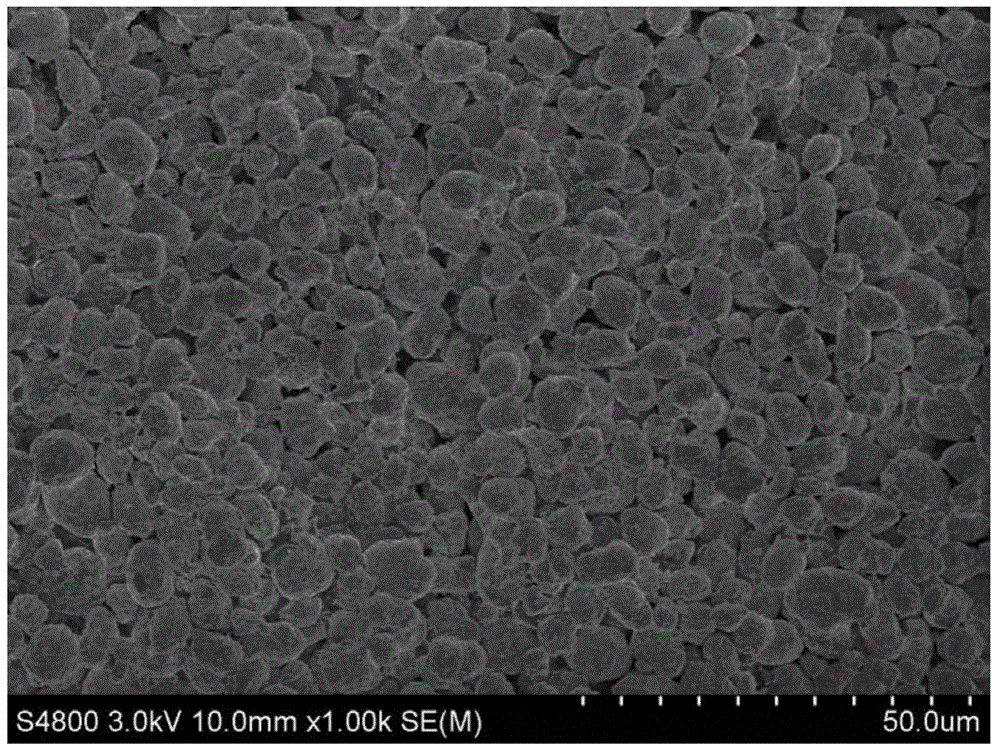
Preparation method of spherical nickel-cobalt-aluminum hydroxide precursor
A spherical nickel-cobalt-aluminum and hydroxide technology, which is applied in nickel oxide/nickel hydroxide, electrical components, battery electrodes, etc., can solve the problem of poor precursor crystal lattice order, uneven distribution of aluminum elements, aluminum ion Fast settling speed and other issues, to achieve the effects of good rate performance and safety performance, high sphericity, and high tap density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Dissolve polyaluminum chloride in deionized water, and mechanically stir until a transparent and clear solution is obtained, and the aluminum ion content is 0.05mol / L to form solution A;
[0041] (2) Nickel sulfate and cobalt sulfate are weighed in a molar ratio of 16:3, then dissolved in deionized water to prepare a salt solution with a mixing uniform concentration of 2mol / L to form solution B;
[0042](3) Pump solution A, solution B, ammonia water and sodium hydroxide together into the co-precipitation reaction kettle equipped with ammonia water and sodium hydroxide mixed solution to carry out continuous co-precipitation reaction (the initial temperature of the bottom liquid is 55 ° C, the pH value 10.5, the ammonia content is 5g / L), the pH value of the reaction system is controlled at 10.5 during the reaction, the ammonia content is controlled at 5g / L in the reaction system, and the temperature of the reaction system is controlled at 55°C; after the reaction is st...
Embodiment 2
[0045] (1) Dissolve polyaluminum chloride in deionized water, and mechanically stir until a transparent and clear solution is obtained, and the aluminum ion content is 0.1mol / L to form solution A;
[0046] (2) Nickel sulfate and cobalt sulfate are weighed in a molar ratio of 17:2, then dissolved in deionized water to prepare a salt solution with a uniform concentration of 1.5mol / L to form solution B;
[0047] (3) Pump solution A, solution B, ammonia and potassium hydroxide together into a co-precipitation reactor equipped with a mixture of ammonia and potassium hydroxide for continuous co-precipitation reaction (the initial temperature of the bottom liquid is 50 ° C, the pH value 12.40, the ammonia content is 25g / L), the pH value of the reaction system is controlled at 12.40 during the reaction, the ammonia content is controlled at 25g / L in the reaction system, and the temperature of the reaction system is controlled at 50°C; after the reaction is stable, the overflowing slurry...
Embodiment 3
[0050] (1) Dissolve polyaluminum chloride in deionized water, and mechanically stir until a transparent and clear solution is obtained, and the aluminum ion content is 0.05mol / L to form solution A;
[0051] (2) nickel sulfate and cobalt sulfate are weighed in a molar ratio of 12:7, then dissolved in deionized water to prepare a salt solution with a uniform concentration of 1.0mol / L to form solution B;
[0052] (3) Pump solution A, solution B, ammonia water and sodium hydroxide together into the co-precipitation reactor equipped with ammonia water and sodium hydroxide mixed solution to carry out continuous co-precipitation reaction (the initial temperature of the bottom liquid is 55 ° C, the pH value 10.70, the ammonia content is 8g / L), the pH value of the reaction system is controlled at 10.70 during the reaction, the ammonia content is controlled at 8g / L in the reaction system, and the temperature of the reaction system is controlled at 50°C; after the reaction is stable, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com