Application of high-positive-charge fluorescent protein in glycosaminoglycans (GAGs) and analogues of GAGs
A glycosaminoglycan and fluorescent protein technology, applied in the field of biochemistry, can solve the problems of low sensitivity, low selectivity, and the application of biocompatibility needs further research, and achieve the effect of overcoming poor biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, the acquisition of ScGFP gene
[0029] Obtain the amino acid sequence of ScGFP (+36) through literature reports [7], and convert the obtained amino acid sequence into the corresponding base sequence, and optimize the codon of the corresponding base to make it easier to be used in E. coli Express. The corresponding DNA sequence was fully synthesized by Sangon Bioengineering Company.
Embodiment 2
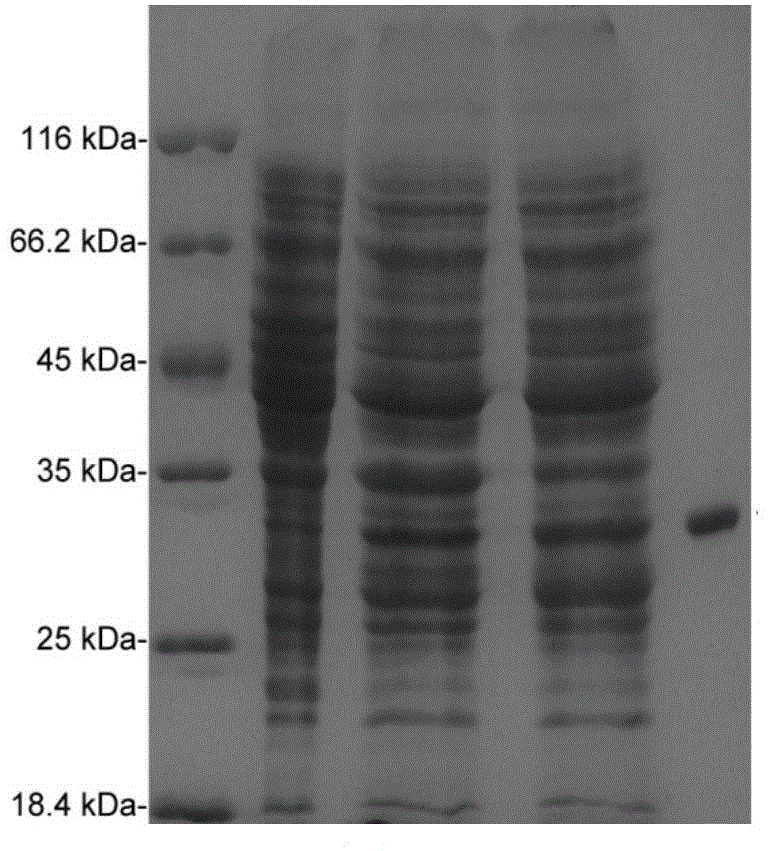
[0030] Embodiment 2, expression and purification of ScGFP (+36) protein
[0031] Using the ScGFP gene obtained in Example 1 as a template, PCR amplification was performed. Primers are as follows:
[0032] Forward primer ScGFP-F:
[0033] (g CATATG ATGGGTCACCATCATCATCACG)
[0034] Reverse primer ScGFP-R:
[0035] (g CTCGAG TTTGTAGCGTTCGTCACGACCGTG)
[0036]The underlined forward primer is the restriction endonuclease Nde I site, and the reverse primer is underlined the restriction endonuclease Xho I site. The PCR product was double-digested, and the double-digested PCR product was ligated with the pET-22b vector that had also been double-digested, and a positive recombinant plasmid (pET22b-ScGFP) was screened. The gene sequencing results showed that the ScGFP gene was inserted between the Nde I and Xho I restriction sites of pET-22b, and the insertion direction was correct.
[0037] pET22b-ScGFP was transformed into Escherichia coli strain BL21(DE3) (purchased from No...
Embodiment 3
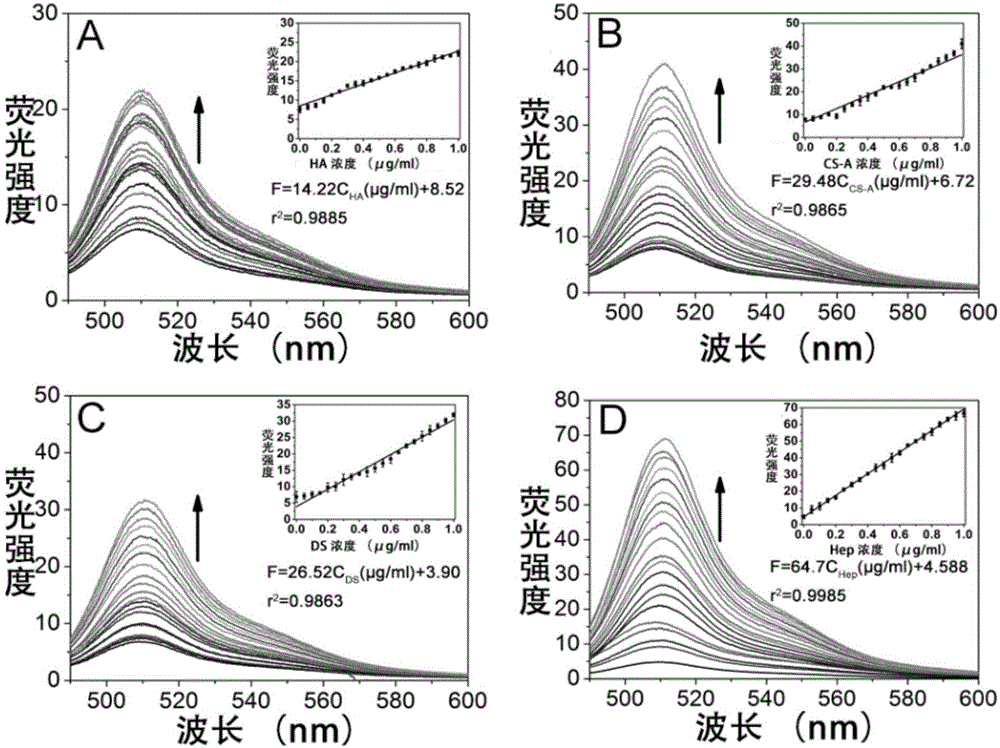
[0038] Example 3. Different concentrations of glycosaminoglycans inhibit the effect of graphene oxide on quenching scGFP fluorescence
[0039] Add 0.01-0.5μg of ScGFP to 10mM Tris-HCl 100mM NaCl (pH7.0) solution, then add heparin (Hep), chondroitin sulfate A (CS-A), dermatan sulfate (DS), hyaluronic acid (HA) to a final concentration of 0-1 μg / ml and a total volume of 190 μl. After standing at room temperature for 10 minutes, add 10 μl of graphene oxide, mix well, and leave at room temperature for 10 minutes. Fluorescence intensity measurement was performed with a fluorescence spectrophotometer (F-4600, Hitachi). The results show that when the concentration of glycosaminoglycan is 0-1 μg / ml, there is a linear relationship between the fluorescence intensity of the reaction system and the concentration of glycosaminoglycan (such as figure 2 ).
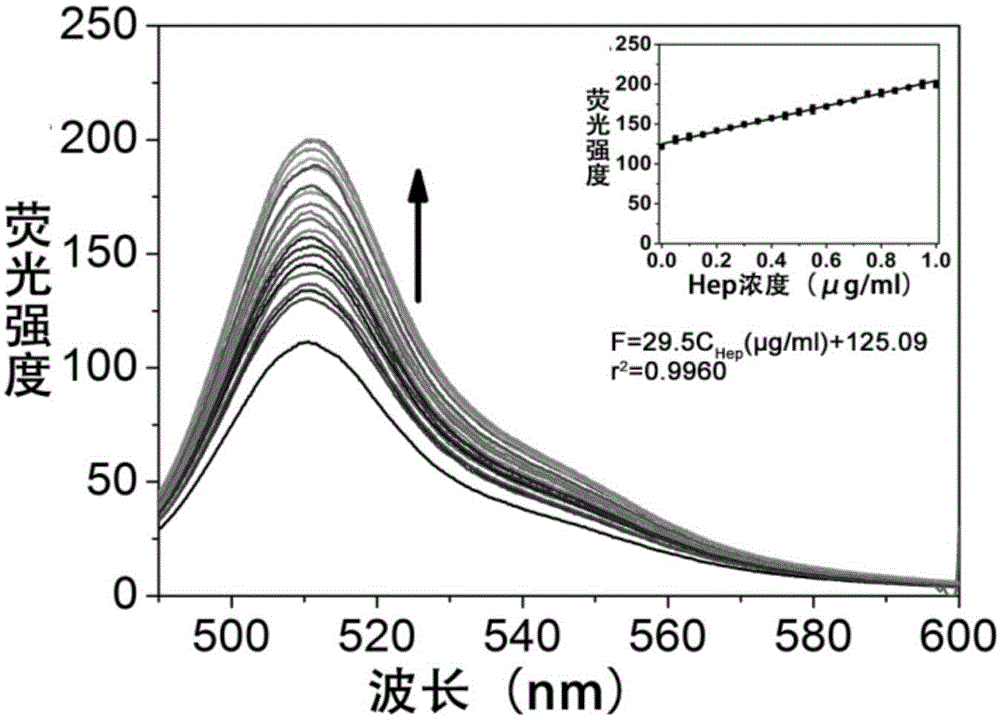
[0040] Add 0.01-0.5 μg of ScGFP to serum diluted 10 times with 10 mM Tris-HCl 100 mM NaCl (pH 7.0), and then add different amounts o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com