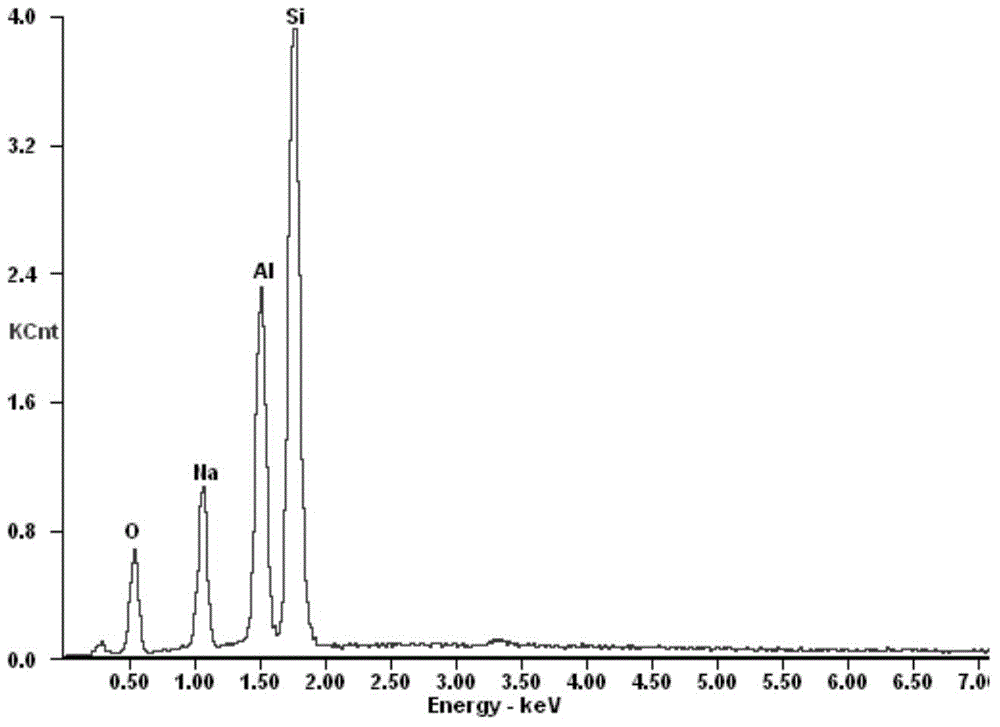
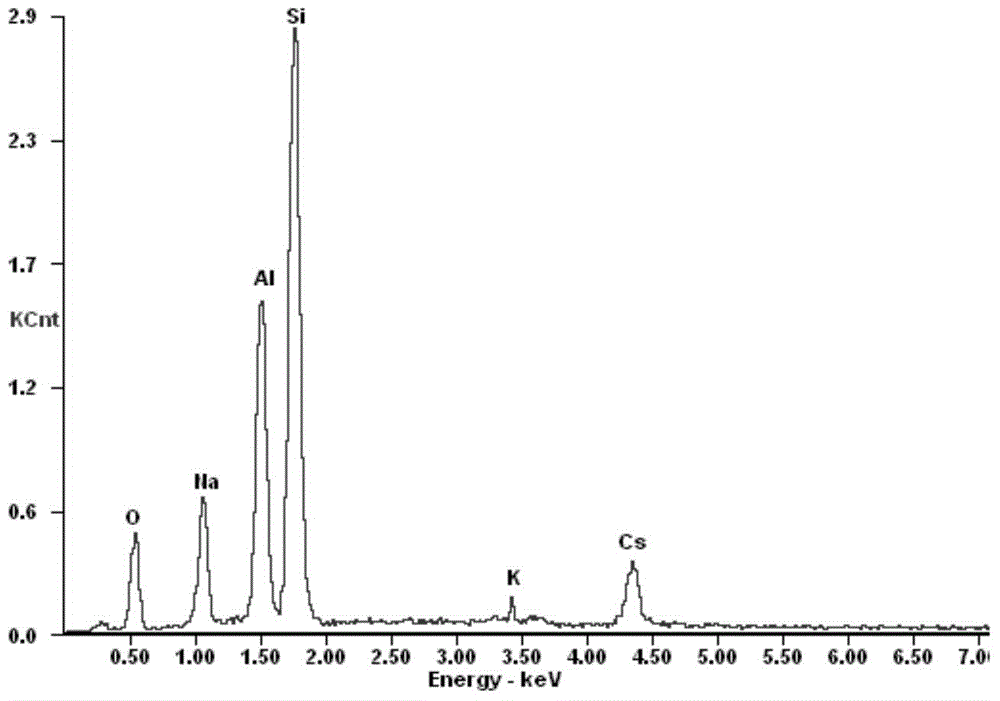
Preparation method of adsorbent for selectively separating cesium ions from salt lake brine
A salt lake brine and adsorbent technology, which is applied in chemical instruments and methods, crystalline aluminosilicate zeolite, other chemical processes, etc., to achieve the effect of no equipment requirements, simple operation, and good adsorption selection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] According to the Si / Al molar ratio being 5.2, prepare 200ml of sodium metaaluminate (NaAlO 2 ) solution and 780ml molar concentration of 0.8M water glass solution (Na 2 SiO 3 9H 20), and in the sodium metaaluminate solution, add 0.6g cetyltrimethylammonium bromide, 1.2g triethanolamine and 6g cesium nitrate successively, stir and fully dissolve to obtain mixed solution A; Then, at room temperature, open Stir, slowly drop the mixed solution A into the prepared water glass solution, and use 10% sodium hydroxide solution to control the pH of the reaction system to be 11.5-12. After the feeding is completed, the above-mentioned feed solution is left to age at room temperature for 48 hours to obtain a silica-alumina gel containing cesium, which is the desired precursor.
[0039] Step 2 Precursor hydrothermal crystallization
[0040] Add the silica-alumina gel obtained above containing cesium into a hydrothermal reaction kettle, and crystallize hydrothermally at 160°C for...
Embodiment 2
[0047] According to the Si / Al molar ratio being 4.8, prepare 200ml of sodium metaaluminate (NaAlO 2 ) solution and 640ml molar concentration of 0.9M water glass solution (Na 2 SiO 3 9H 2 0), and in the sodium metaaluminate solution, add 0.5g cetyltrimethylammonium bromide, 1g triethanolamine and 12g cesium nitrate successively, stir and fully dissolve to obtain mixed solution A; Then, at room temperature, start stirring , slowly drop the mixed solution A into the prepared water glass solution, and use 10% potassium hydroxide solution to control the pH of the reaction system to be 11.5-12. After the feeding is completed, the above-mentioned feed solution is left to age at room temperature for 48 hours to obtain a silica-alumina gel containing cesium, which is the desired precursor.
[0048] Step 2 Precursor hydrothermal crystallization
[0049] Add the silica-alumina gel obtained above containing cesium into a hydrothermal reaction kettle, and crystallize it hydrothermally ...
Embodiment 3
[0056] According to the Si / Al molar ratio being 4.2, prepare 200ml of sodium metaaluminate (NaAlO 2 ) solution and 560ml molar concentration of 0.6M water glass solution (Na 2 SiO 3 9H 2 0), and in the sodium metaaluminate solution, add 0.4g cetyltrimethylammonium bromide, 0.8g triethanolamine and 3g cesium nitrate successively, stir and fully dissolve to obtain mixed solution A; Then, at room temperature, open Stir, slowly drop the mixed solution A into the prepared water glass solution, and use 12% lithium hydroxide solution to control the pH of the reaction system to be 11.5-12. After the feeding is completed, the above-mentioned feed solution is left to age at room temperature for 48 hours to obtain a silica-alumina gel containing cesium, which is the desired precursor.
[0057] Step 2 Precursor hydrothermal crystallization
[0058] Add the silica-alumina gel obtained above containing cesium into a hydrothermal reaction kettle, and crystallize hydrothermally at 170°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com