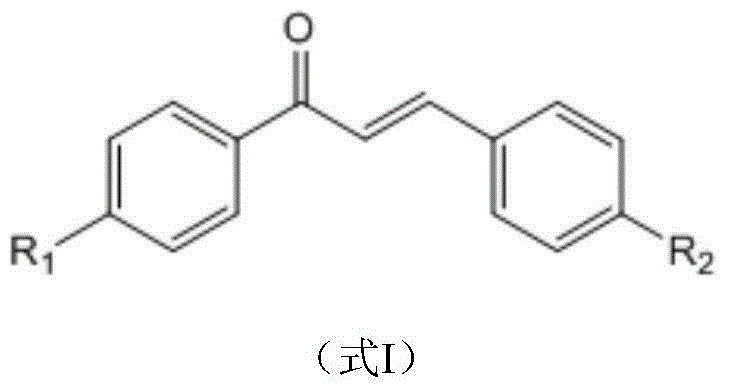
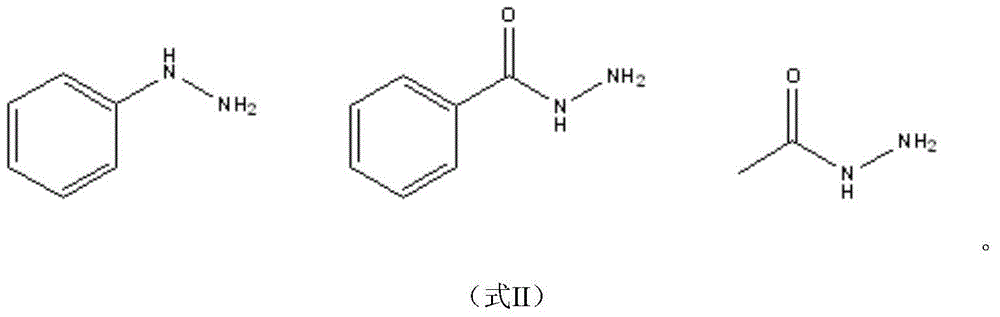
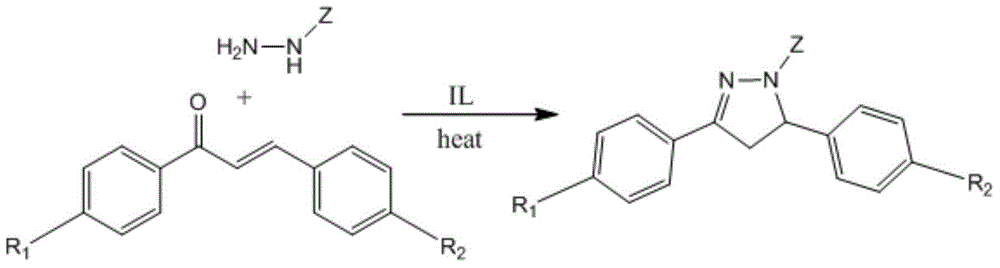
Environment-friendly method for catalytic synthesis of 1,3,5-triple substituent-2-pyrazoline derivative through ionic liquid
An ionic liquid and three-substitution technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as low yield, long reaction time, complicated post-processing, etc., and achieve reduced usage , reduced reaction time, and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 1-sulfonic acid butyl-3-methylimidazolium triflate:
[0025] Take 6.8g (0.05mol) of 1,4-sultone and 4.0g (0.05mol) of N-methylimidazole in a round bottom flask, stir at room temperature for 24 hours, and wash the solid product with diethyl ether and toluene to remove residual The raw material impurities of the reaction were then vacuum-dried, then added 7.5g (0.05mol) trifluoromethanesulfonic acid and stirred at 40°C for 2h to generate 1-sulfonic acid butyl-3-methylimidazole trifluoromethanesulfonate, liquid phase The product was repeatedly washed with toluene and ether to remove non-ionic impurities, and then vacuum-dried to obtain a relatively pure ionic liquid product.
[0026] The physical properties and spectral data of the obtained compound are as follows: δ H 9.161(s,1H),7.78(s,1H),7.748(s,1H),4.210(t,J=7.2,2H),3.89(s,3H),2.68(t,J=7.2,2H), 1.90 (q, J = 7.6, 2H), 1.61 (q, J = 4.6, 2H).
Embodiment 2
[0028] 1,3-Diphenyl-5-(4-dimethylaminophenyl)-2-pyrazoline
[0029] Take 0.25g (1mmol) 1-phenyl-3-(4-dimethylaminophenyl)-2-propenyl-1-one, 0.14g (1.3mmol) phenylhydrazine and 2mL ionic liquid 1-sulfonic acid butyl -3-Methylimidazole trifluoromethanesulfonate was dissolved in a two-necked round-bottomed flask by ultrasonic vibration, connected to a reflux device, and stirred at a temperature of 50°C for 0.5h (TLC tracking and monitoring of the reaction), the reaction solution was cooled and filtered with suction An orange-yellow solid was obtained, which was then recrystallized from ethanol to obtain 1,3-diphenyl-5-(4-dimethylaminophenyl)-2-pyrazoline as yellow needle crystals. The yield was 0.30 g, and the yield was 89.4%. .
[0030] The physical properties and spectral data of the obtained compound are as follows: mp 108-109°C, δ H 7.72(d,J=6.0Hz,2H),7.32~7.44(m,3H),7.00~7.16(m,6H),6.64~6,71(m,3H),5.29(dd,J 1 =6.0Hz,J 2 =12.0Hz,1H),3.78(dd,J 1 =12.0Hz,J 2 =18.0Hz,1H),...
Embodiment 3
[0032] 1-phenyl--3-(4-methylphenyl)-5-(3-azidophenyl)-2-pyrazoline
[0033] Take 0.27g (1mmol) 1-(4-methylphenyl)-3-(3-azidophenyl)-2-propenyl-1-one, 0.14g (1.3mmol) phenylhydrazine and 2mL ionic liquid 1-Sulphonic acid butyl-3-methylimidazolium trifluoromethanesulfonate was dissolved in a two-necked round-bottomed flask by ultrasonic vibration, connected to a reflux device, and the temperature was controlled at 50°C to stir and react for 1 h (TLC tracking and monitoring the reaction), and the reaction Liquid cooling, suction filtration to obtain orange solid powder, and then recrystallized with ethanol to obtain orange needle-like crystal 1-phenyl-3-(4-methylphenyl)-5-(3-azidophenyl) -2-pyrazoline, yield 0.31 g, yield 85.0%.
[0034] The physical properties and spectral data of the obtained compound are as follows: mp 124-126°C, δ H 7.61(d, J=9.0Hz, 2H), 7.37(t, J=9.0Hz, 1H), 6.97~7.24(m, 9H), 6.71(t, J=6.0Hz, 1H), 5.44(dd,J 1 =6.0Hz,J 2 =12.0Hz,1H),3.83(dd,J 1 =12.0Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com