Prussian blue analogous positive material for sodium-ion batteries and preparation method of positive material
A technology for sodium ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, non-aqueous electrolyte batteries, etc., can solve the problems of poor cycle performance and low capacity, achieve preparation methods and are easy to control, high capacity, and materials The effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
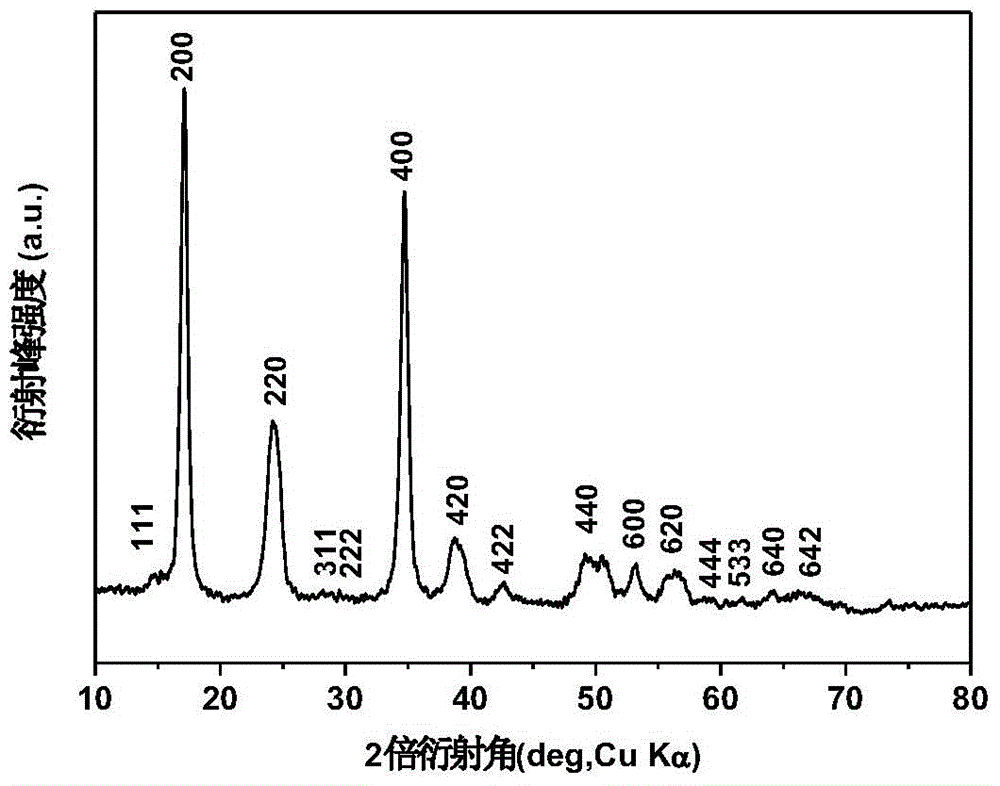
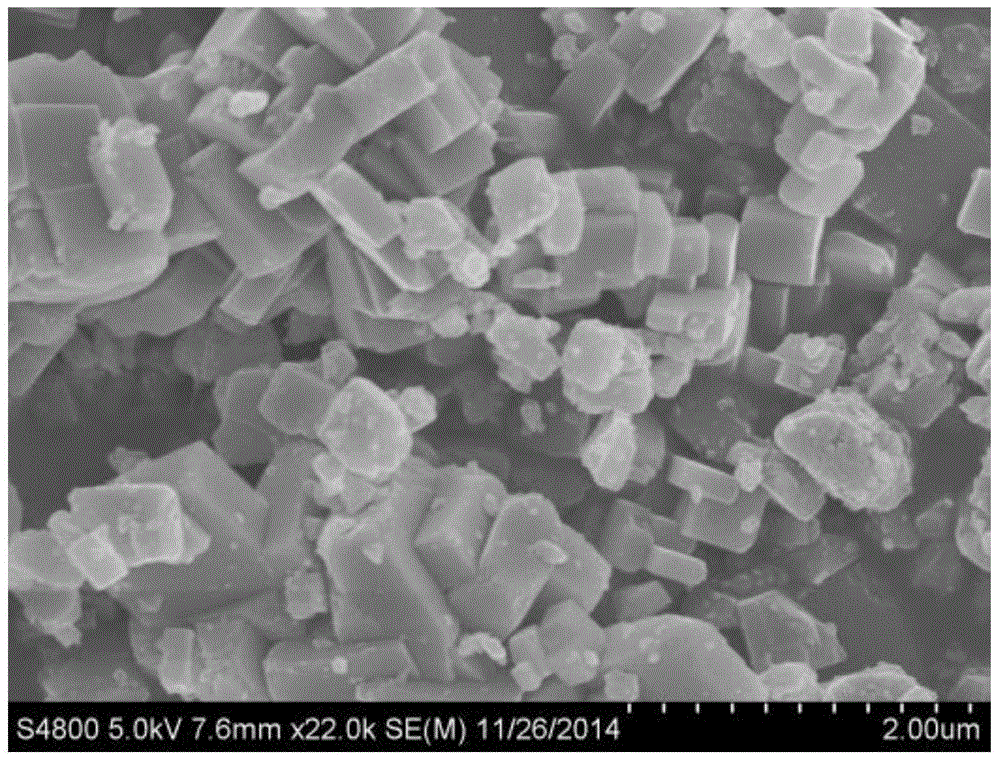
Image
Examples
Embodiment 1
[0037] Step 1, liquid phase precipitation reaction
[0038] (1) Weigh 0.951g of nickel dichloride hexahydrate (NiCl 2 ·6H 2 0) add in 40mL deionized water, stir to make it dissolve, obtain nickel chloride solution concentration and be 0.1mol / L; Take by weighing 1.428g cobalt dichloride hexahydrate (CoCl 2 ·6H 2 0) add in 60mL deionized water, stir to make it dissolve, obtain cobalt chloride solution concentration and be 0.1mol / L;
[0039] (2) Weigh 4.841g of sodium ferrocyanide decahydrate (Na 4 Fe(CN) 6 10H 2 O) add in 100mL deionized water, stir to make it dissolve, obtain mother liquor concentration and be 0.1mol / L;
[0040] (3) 40mL of nickel salt solution and 60mL of cobalt salt solution were dropped into the mother liquor at the same time with a peristaltic pump to obtain a mixed solution, and the mixed solution was continuously stirred and reacted for 6 hours at room temperature and protected from light;
[0041] (4) Aging the mixed solution after stirring and re...
Embodiment 2
[0046] Step 1, liquid phase precipitation reaction
[0047] (1) Weigh 0.0951g of NiCl 2 ·6H 2 Add O into 40mL deionized water, stir to dissolve it, and obtain a nickel chloride solution with a concentration of 0.01mol / L; weigh 0.1428g of CoCl 2 ·6H 2 O was added in 60mL of deionized water, stirred and dissolved to obtain a concentration of cobalt chloride solution of 0.01mol / L;
[0048] (2) Weigh 0.4841g of Na 4 Fe(CN) 6 10H 2 O was added in 100mL of deionized water, stirred to make it dissolve, and the mother liquor concentration obtained was 0.01mol / L;
[0049] (3) 40mL of nickel salt solution and 60mL of cobalt salt solution were added dropwise to the mother liquor with a peristaltic pump at the same time to obtain a mixed solution, and the mixed solution was continuously stirred and reacted for 4 hours at room temperature and protected from light;
[0050] (4) Aging the mixed solution after stirring and reacting for 24 hours at room temperature under the condition o...
Embodiment 3
[0055] Step 1, liquid phase precipitation reaction
[0056] (1) Weigh 1.051g of nickel sulfate hexahydrate (NiSO 4 ·6H 2 0) add in 40mL deionized water, stir to make it dissolve, obtain nickel sulfate solution concentration and be 0.1mol / L; Take by weighing 1.687g cobalt sulfate heptahydrate (CoSO 4 ·7H 2 0) add in 60mL deionized water, stir to make it dissolve, obtain cobalt sulfate solution concentration and be 0.1mol / L;
[0057] (2) Weigh 4.841g of Na 4 Fe(CN) 6 10H 2 O) add in 100mL deionized water, stir to make it dissolve, obtain mother liquor concentration and be 0.1mol / L;
[0058] (3) 40mL of nickel salt solution and 60mL of cobalt salt solution were dropped into the mother liquor at the same time with a peristaltic pump to obtain a mixed solution, and the mixed solution was continuously stirred and reacted at room temperature and protected from light for 6h;
[0059] (4) Aging the mixed solution after stirring and reacting for 72 hours at room temperature under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com