Method for ammonia-process capture of carbon in flue gas and synthesis of chemical products
A chemical product and carbon capture technology, applied in the field of flue gas purification, can solve the problems that cannot be directly and effectively used, and achieve the effect of reducing carbon emissions, reducing emission values, and increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
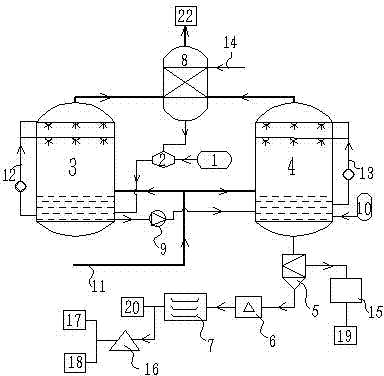
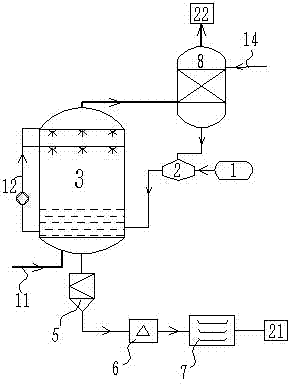
[0053] like figure 2 As shown, the flue gas generated by the power plant goes through processes such as dust removal and desulfurization, and all the flue gas enters the bottom of the absorption tower 3 . Liquid ammonia enters into dilution tank 2 from ammonia water tank 1 and is diluted into 10% ammonia water. The ammonia water is pre-added into absorption tower 3 to react with the flue gas entering from the bottom. Ammonia water passes through absorption liquid circulation pipeline 12 to the spraying device, and the ammonia water is sprayed to further Decarbonize the escaping flue gas. When the pH of the absorption liquid drops to 9.3, the ammonia water tank 1 is opened to add ammonia to the absorption tower 3, and when the pH is close to 12, the ammonia water tank 1 is closed to stop adding ammonia. Monitor the pH value in the absorption tower and maintain the pH in the absorption liquid within the range of 9.3-11.5 by adjusting the amount of ammonia added. Until the rea...
Embodiment 2
[0055] like figure 1 As shown, the concentrated ammonia water enters the dilution tank 2 from the ammonia water tank 1 and is diluted into 15% ammonia water, and the ammonia water is pre-added in the absorption tower 3, and the flue gas passes through operations such as dedusting, desulfurization, and 70% of the flue gas enters the side wall of the absorption tower 3, The position where the flue gas is introduced is above the liquid level. The ammonia water passes through the absorption liquid circulation pipeline 12 to the spraying device, and the carbon dioxide in the flue gas is removed by the reverse convection of the ammonia water and the flue gas by spraying. The decarbonization efficiency of the absorption tower is 82%. When the pH of the absorption liquid drops to 9.3, the ammonia water tank 1 is opened to add ammonia to the absorption tower 3, and the pH reaches 11.5, and the ammonia water tank 1 is closed to stop adding ammonia. Monitor the pH value in the absorptio...
Embodiment 3
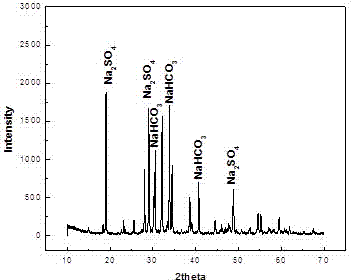
[0059] The reaction in the carbonization tower was simulated under the experimental conditions. The carbonization tower contained 1 L of ammonium carbonate solution with a concentration of 7.25 mol / L. The solution contained ammonia water with a concentration of 2%, and sodium sulfate particles were added to the solution until the solution was saturated. Pass into the flue gas, in which the liquid-gas ratio (L / G) is 0.40, in which the CO 2 The partial pressure is 45KPa. The spray amount of the intermediate mother liquor was 3L / min. The pH of the carbonization mother liquor was in the range of 8.5-9.5, and the reaction temperature was 40°C. Continuous production is carried out to obtain the product sodium bicarbonate. The final conversion rate of carbon is 34.23% (the conversion rate of carbon is the ratio of the product to the carbon content of the flue gas entering the tower), of which CO 2 The absorption efficiency is about 26%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com