Medicinal composition for improving stability of crystal medicines, and preparation method thereof
A technology of composition and stability, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of not being able to guarantee the stability of crystal I, and accelerate the crystal formation of agomelatine Transformation, narrow choice of accessories, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
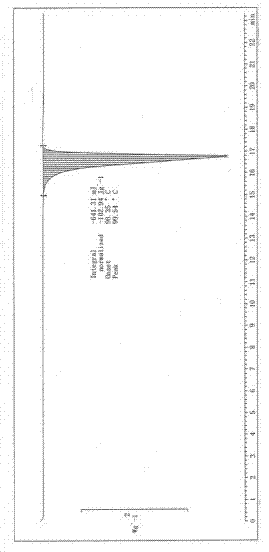
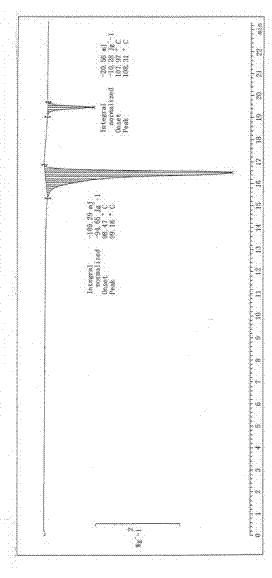
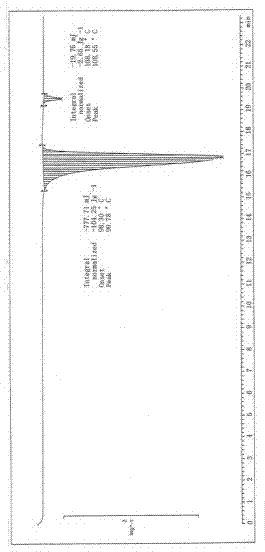
Image
Examples
Embodiment 1
[0152] Agomelatine (Crystal Form I 99%) 25g
[0153] water 20ml
[0154] Lactose 102g
[0155] Hydroxypropyl Cellulose 3g
[0156] Polyvinylpyrrolidone k30 3g
[0157] Croscarmellose Sodium 13g
[0158] Magnesium Stearate 1.3g
[0159] Stearic acid 2.6g
[0160] Silica 0.3g
[0161] Process: Sieve the crystalline form I agomelatine according to the above weight for later use; take hydroxypropyl cellulose and polyvinylpyrrolidone k30 and stir to dissolve in water, then add the crystalline form I agomelatine and stir evenly to obtain the protected The crystalline form I agomelatine of the agent is ready for use; then lactose, part (1 / 2) of croscarmellose sodium is added to the wet mixing granulator and mixed, and then the crystalline form I containing the protective agent is added. Agomelatine, granulated for 2 minutes, granulated by a oscillating granulator (833 μm aperture sieve); fluidized bed drying (inlet air temperature 45°C, boiling bed temperature 35°C), granulate...
Embodiment 2
[0163] Tenofovir (more than 90% crystalline form I) 300g
[0164] water 450ml
[0165] Hypromellose 15g
[0166] Polyvinylpyrrolidone k30 15g
[0167] Lactose 120g
[0168] Microcrystalline Cellulose 40g
[0169] 25g pregelatinized starch
[0170] Cross-linked polyvinylpyrrolidone 13g
[0171] Magnesium Stearate 1.3g
[0172] Process: sieve the crystalline type I tenofovir according to the above weight for later use; take hydroxypropyl methylcellulose and polyvinylpyrrolidone k30 in water and stir to dissolve, then add the crystalline type I tenofovir and stir well; obtain the protective agent containing The crystalline type I tenofovir is ready for use; then add lactose, microcrystalline cellulose, pregelatinized starch, and part (1 / 2) cross-linked polyvinylpyrrolidone into the wet mixing granulator and mix well, and then add protective Crystal type I tenofovir, granulated for 2min, granulated by a swing granulator (833μm aperture sieve); fluidized bed drying (inlet ai...
Embodiment 3
[0174] Adefovir dipivoxil (more than 85% crystal type I) 10g
[0175] water 50ml
[0176] Hypromellose 5g
[0177] Mannitol 115g
[0178] Microcrystalline Cellulose 39g
[0179] Sodium carboxymethyl starch 8g
[0181]Process: sieve crystal I adefovir dipivoxil according to the above weight for later use; take hydroxypropyl methylcellulose and stir and dissolve it in water, add crystal I type adefovir dipivoxil and stir evenly; obtain crystal I type containing protective agent Adefovir dipivoxil is ready for use; then add mannitol, microcrystalline cellulose, and part (1 / 2) sodium carboxymethyl starch into the wet mixing granulator and mix well, then add crystal I Adefovir containing protective agent Granulate, granulate for 2 minutes, granulate through a oscillating granulator (833μm sieve); fluidized bed drying (inlet air temperature 45°C, boiling bed temperature 35°C), granulate, add the rest of the excipients in proportion and mix we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com