Fetal bovine marrow tablet and production method thereof
A technology of bone marrow tablets and fetal bovine, applied in the field of fetal bovine bone marrow tablets and its production, can solve the problems of low utilization rate of active substances, achieve the effects of improving immunity, enhancing immune function, and preventing colds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
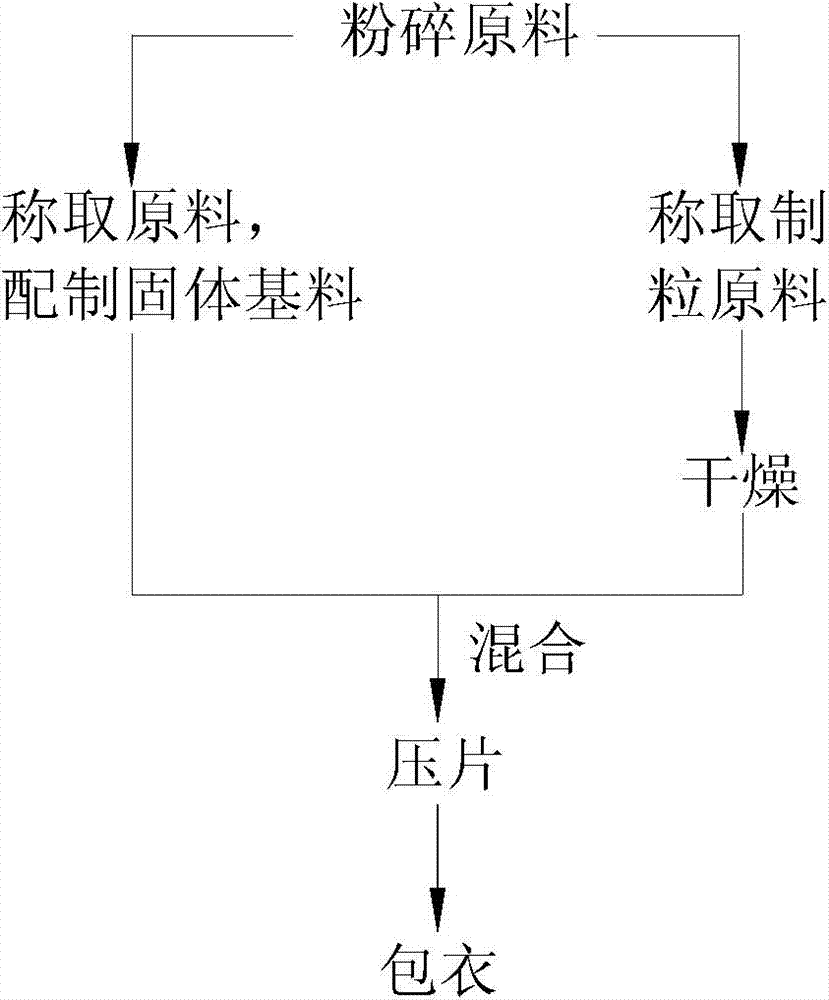
[0026] like figure 1 Shown, the present invention proposes a kind of method of producing fetal bovine bone marrow sheet, comprises steps:
[0027] 1) Pulverize the raw materials, and then sieve them through a 200-mesh sieve to screen fine particles;
[0028] 2) Take 3.5 parts of bone peptide, 6.2 parts of enzymatic bone calcium powder, 13.4 parts of chrysanthemum powder, 0.01 part of zinc lactate, 2.6 parts of calcium carbonate, 2.5 parts of brewer's yeast, 3.8 parts of selenium-enriched yeast, 4.1 parts of cornstarch, and mix well Cross 60 mesh sieves to make solid base material;
[0029] 3) Weighing 4 parts of fetal bovine bone marrow, adding 1 part of 50% ethanol solution to the fetal bovine bone marrow and stirring evenly to obtain wet granules;
[0030] 4) The wet granules are dried for 60 minutes at 50° C. and a relative humidity of 30% to form dry granules;
[0031] 5) Mix the dry granules with the solid base material, then add 0.2 parts of magnesium stearate and 0.2...
Embodiment 2
[0034] like figure 1 Shown, the present invention proposes a kind of method of producing fetal bovine bone marrow sheet, comprises steps:
[0035] 1) Pulverize the raw materials, and then sieve them through a 200-mesh sieve to screen fine particles;
[0036] 2) Take 5.2 parts of bone peptide, 8.9 parts of enzymatic bone calcium powder, 16.7 parts of chrysanthemum powder, 0.05 parts of zinc lactate, 10.2 parts of calcium carbonate, 7.3 parts of brewer's yeast, 9.6 parts of selenium-enriched yeast, 10.3 parts of cornstarch, and mix well Cross 60 mesh sieves to make solid base material;
[0037] 3) Weigh 5.2 parts of fetal bovine bone marrow, add 2 parts of 50% ethanol solution to the fetal bovine bone marrow and stir evenly to obtain wet granules;
[0038] 4) The wet granules are dried for 60 minutes at 50° C. and a relative humidity of 30% to form dry granules;
[0039] 5) Mix the dry granules with the solid base material, then add 0.8 parts of magnesium stearate and 0.6 par...
Embodiment 3
[0042] like figure 1 Shown, the present invention proposes a kind of method of producing fetal bovine bone marrow sheet, comprises steps:
[0043] 1) Pulverize the raw materials, and then sieve them through a 200-mesh sieve to screen fine particles;
[0044] 2) Take 10.1 parts of bone peptide, 20.7 parts of enzymatic bone calcium powder, 19 parts of chrysanthemum powder, 0.08 parts of zinc lactate, 15.6 parts of calcium carbonate, 14.2 parts of brewer's yeast, 16.4 parts of selenium-enriched yeast, 17.8 parts of cornstarch, and mix well Cross 60 mesh sieves to make solid base material;
[0045] 3) Weigh 7.6 parts of fetal bovine bone marrow, add 3 parts of 50% ethanol solution to the fetal bovine bone marrow and stir evenly to obtain wet granules;
[0046] 4) The wet granules are dried for 60 minutes at 55° C. and a relative humidity of 35% to form dry granules;
[0047] 5) Mix the dry granules with the solid base material, then add 1.8 parts of magnesium stearate and 1.3 par...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com