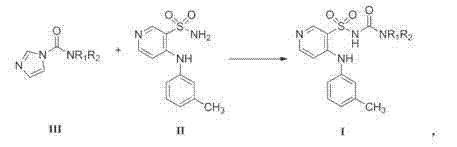
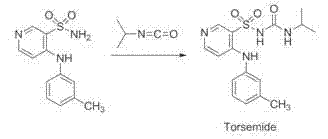
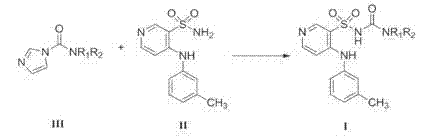
Method for preparing torasemide and derivative thereof
A technology of torasemide and derivatives, applied in the field of drug synthesis, can solve the problems of high price, skin irritation, high toxicity of isopropyl isocyanate, etc., and achieve production capacity improvement, reaction time shortening, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1N-ethyl-[4-(3-methylphenylamino)pyridine-3-sulfonamido]formamide
[0020] Add 500mL ethanol to a 1L three-necked flask, add N-ethyl-1H-imidazole-1-carboxamide (69.58g, 0.50mol), 4-(3-methylphenylamino)pyridine-3-sulfonamide (105.3g , 0.40mol), and mix well under stirring. Heat to reflux, react for 7-8 hours, distill under reduced pressure, and concentrate to dryness. Add 125mL of concentrated ammonia water (25-28%) and 500mL of purified water to the residue, heat and dissolve under stirring, and after complete clarification, add 1.0g of activated carbon, keep warm and decolorize and stir for 15-30min. Filtrate while it is hot, cool the filtrate to 40-50°C, neutralize the filtrate with glacial acetic acid to pH=6-7, cool to 10-20°C, crystallize for 4 hours and then filter. The filter cake was washed twice with purified water. The wet product is air-dried at 60-65°C for 6-8 hours. Weigh, weight is 80g, and yield is 60.0%. ESI-MS(m / z): 3...
Embodiment 2
[0021] The preparation of embodiment 2N-ethyl-[4-(3-methylphenylamino)pyridine-3-sulfonamido]formamide
[0022] Add 500mL ethanol to a 1L three-necked flask, add N-ethyl-1H-imidazole-1-carboxamide (69.58g, 0.50mol), 4-(3-methylphenylamino)pyridine-3-sulfonamide (105.3g , 0.40mol) and pyridine (79.1g, 1.0mol), mix well under stirring. Heat to reflux and react for 4-6 hours. TLC monitoring, after the completion of the reaction, vacuum distillation, concentrated to dryness. Add 125mL of concentrated ammonia water (25-28%) and 500mL of purified water to the residue, heat and dissolve under stirring, and after complete clarification, add 1.0g of activated carbon, keep warm and decolorize and stir for 15-30min. Filtrate while it is hot, cool the filtrate to 40-50°C, neutralize the filtrate with glacial acetic acid to pH=6-7, cool to 10-20°C, crystallize for 4 hours and then filter. The filter cake was washed twice with purified water. The wet product is air-dried at 60-65°C for ...
Embodiment 3
[0023] The preparation of embodiment 3N-isopropyl-[4-(3-methylphenylamino)pyridine-3-sulfonamido]formamide
[0024] Add 500mL of acetone into a 1L three-necked flask, add N-isopropyl-1H-imidazole-1-carboxamide (76.60g, 0.50mol), 4-(3-methylphenylamino)pyridine-3-sulfonamide (105.3 g, 0.40mol) and triethylamine (101.2g, 1.0mol), mix well under stirring. Heat up to 40-50°C and react for 4-6 hours. TLC monitoring, after the reaction is complete, distill under reduced pressure and concentrate to dryness. Add 125mL of concentrated ammonia water (25-28%) and 500mL of purified water to the residue, heat and dissolve under stirring, and after complete clarification, add 1.0g of activated carbon, keep warm and decolorize and stir for 15-30min. Filtrate while it is hot, cool the filtrate to 40-50°C, neutralize the filtrate with glacial acetic acid to pH=6-7, cool to 10-20°C, crystallize for 4 hours and then filter. The filter cake was washed twice with purified water. The wet produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com