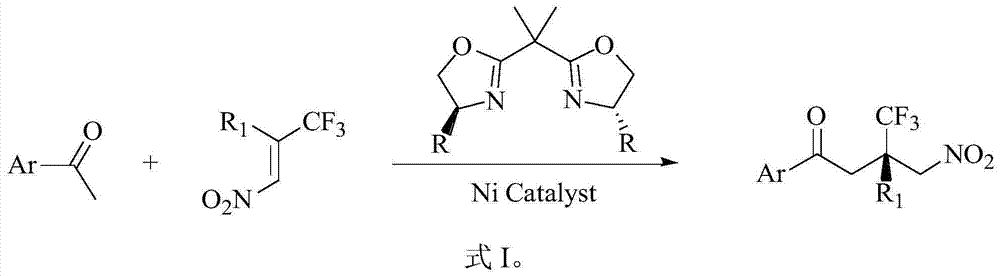
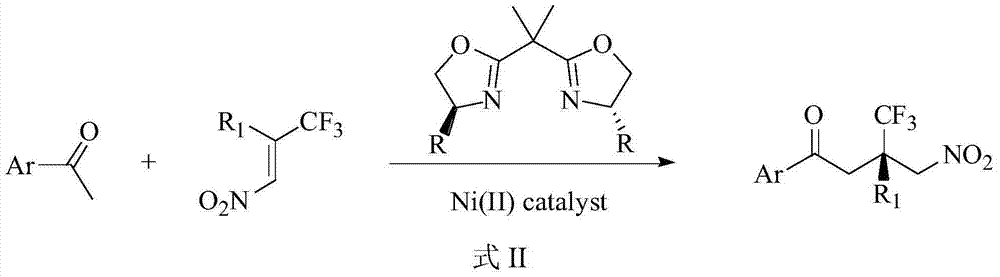
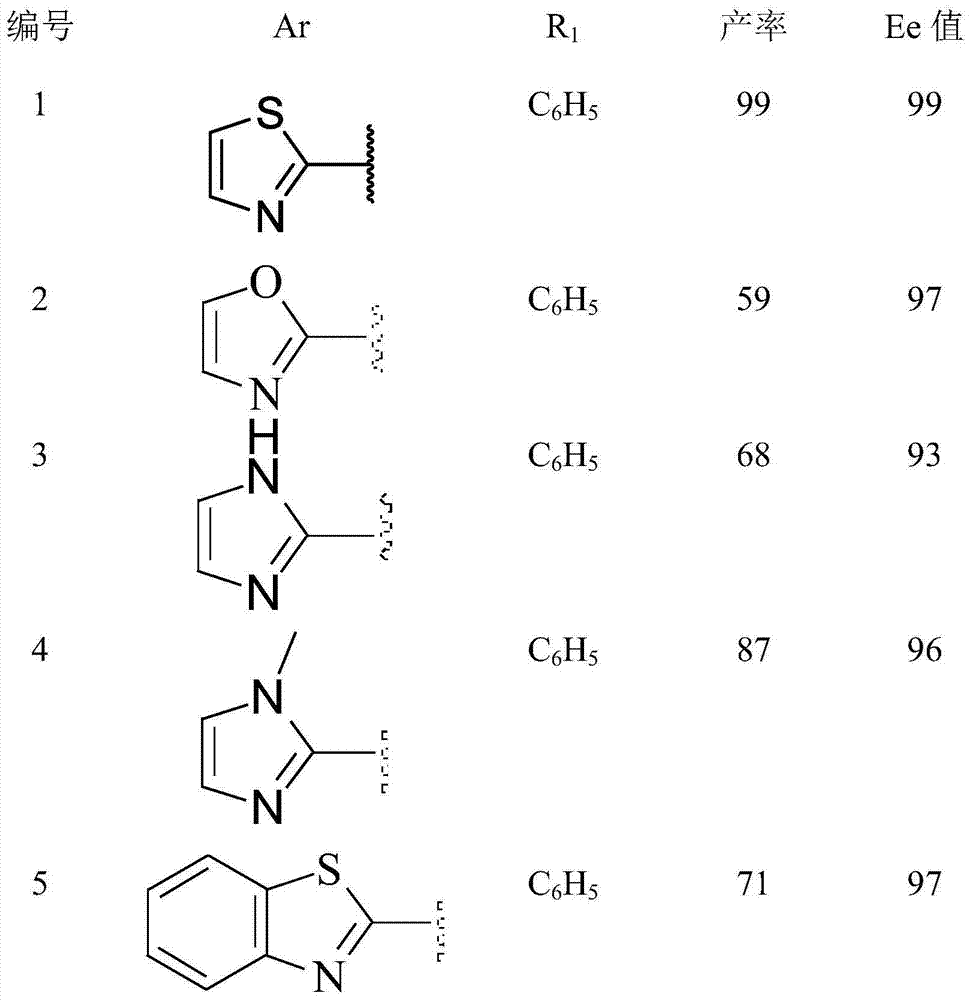
Method for asymmetrically synthesizing chiral quaternary carbon compound containing trifluoromethyl
A technology of trifluoromethyl and carbon compounds, applied in organic chemistry and other directions, can solve the problems of low yield, non-reaction, poor reaction activity, etc., and achieve the effect of high reaction yield and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of 2-trifluoromethyl-2-phenyl-1-nitroethene:
[0032] Add lithium trifluoroacetate (3g, 25mmol) and magnesium chips (0.6g, 25mmol) to a dry 100mL single-necked bottle, replace the N 2 Three times, add THF (tetrahydrofuran 20mL) under ice bath, stir for 5min and then add bromobenzene (4.71g, 30mmol) dropwise. After the reaction is complete, add 10 mL of aqueous HCl solution with a concentration of 1 mol / L to the reaction flask, stir for 5 minutes and then filter with suction. After adding water, extract the obtained filtrate with 3×50 mL of ethyl acetate, combine the organic phases and wash with concentrated brine, anhydrous sodium sulfate After drying and desolvation, the crude product was obtained, which was separated by silica gel column (volume ratio ethyl acetate:petroleum ether=1:50) to obtain yellow liquid trifluoromethyl phenyl ketone (0.87g, 20% yield).
[0033] Add trifluoromethyl phenyl ketone (1.25g, 7.18mmol) to a 100mL single-necked bottle, then ...
Embodiment 2
[0036] Synthesis of (R)-3-trifluoromethyl-4-nitro-3-phenyl-1-(2-thiazole)-1-butanone:
[0037]
[0038] Add bisoxazoline ligand 9.2mg (0.0275mmol) to a dry 10mL round bottom flask, Ni(AcAc) 2 (6.5mg, 0.0254mmol), add 1mL of isopropanol under nitrogen protection, stir at room temperature for 30min, then add 2-acetylthiazole (32.4mg, 0.25mmol) dissolved in 0.2mL of isopropanol, and stir at 0°C for 30min , add 0.3 mL of 2-trifluoromethyl-2-phenyl-1-nitroethylene dissolved in isopropanol. After adding the sample, react at 0°C for 24h. After the reaction was completed, the reaction mixture was directly separated and purified by silica gel chromatography (ethyl acetate:petroleum ether=1:10, volume ratio) after desolvation under reduced pressure to obtain a white solid 3-trifluoromethyl-4-nitro- 3-Phenyl-1-(2-thiazole)-1-butanone 81.8 mg (99% yield).
Embodiment 3
[0040] (R)-3-trifluoromethyl-3-phenyl-4-nitro-1-(2-pyridine)-1-butanone:
[0041]
[0042] Add bisoxazoline ligand 9.2 mg (0.0275 mmol), Ni(AcAc) to a dry 10 mL round bottom flask 2 (6.5 mg, 0.0254 mmol), add 1 mL of isopropanol, stir at room temperature for 30 minutes under nitrogen protection, then add 2-acetylpyridine (30.25 mg, 0.25 mmol) dissolved in 0.2 mL of isopropanol, and stir at 0 ° C for 30 minutes , 2-trifluoromethyl-2-phenyl-1-nitroethylene (81.4 mg, 0.375 mmol) dissolved in 0.3 mL of isopropanol was added. After adding the sample, react at 0°C for 24h. After the reaction was completed, the reaction mixture was directly separated and purified by silica gel chromatography (ethyl acetate:petroleum ether=1:4, volume ratio) after desolvation under reduced pressure to obtain a white solid 3-trifluoromethyl-4-nitro- 3-Phenyl-1-(2-pyridine)-1-butanone 80.3 mg (95% yield).
[0043] m.p.: 148-149°C; [α]=–16.24 (c=0.46, CH 2 Cl 2 ); 92%ee, determined by HPLC analys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com