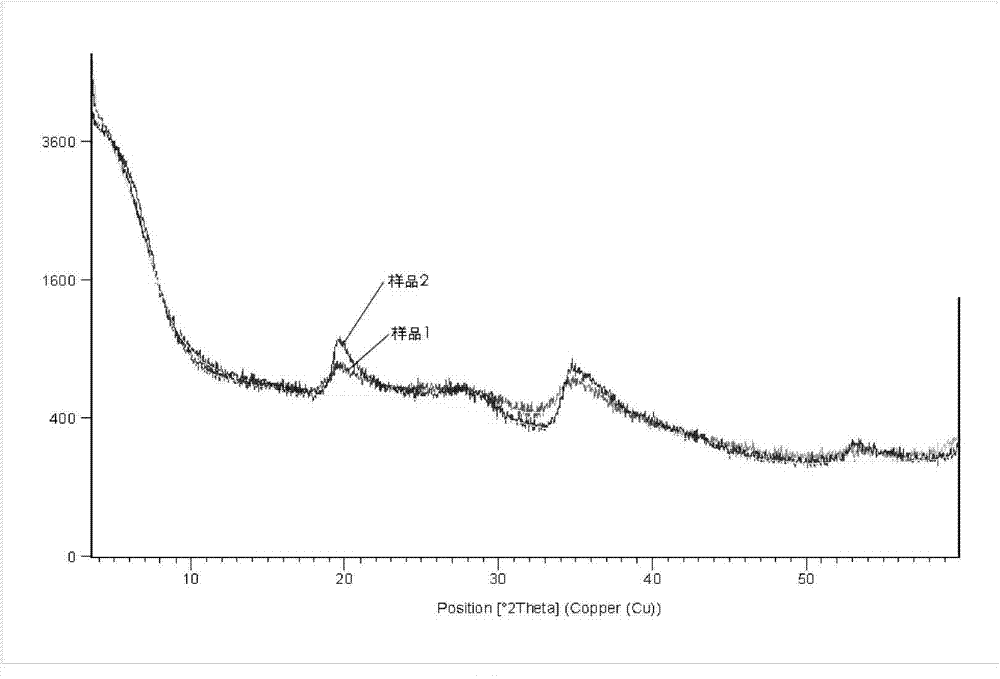
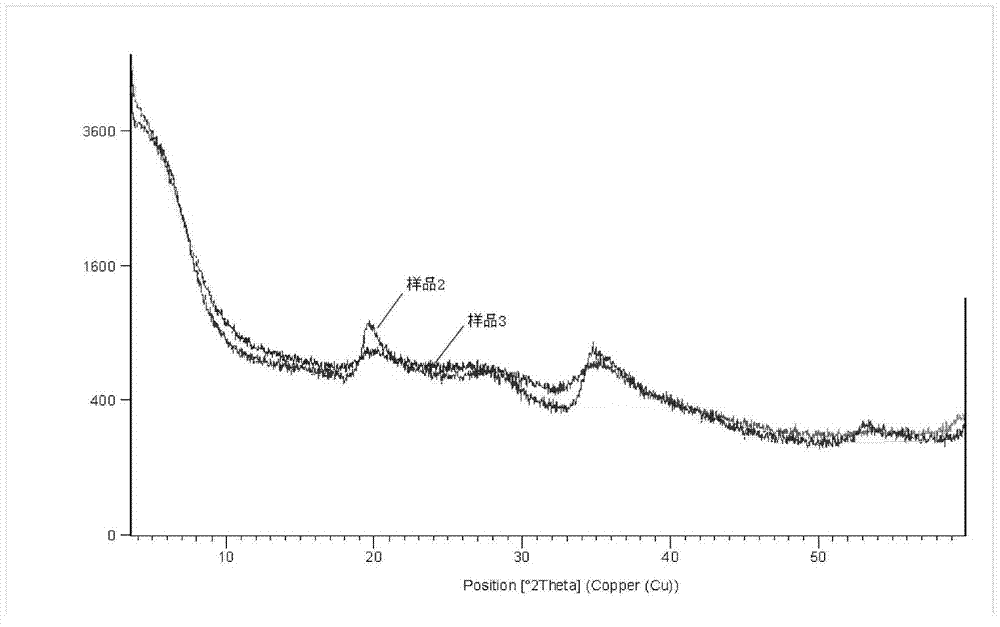
Method for mechanochemically synthesizing magnesium lithium silicate
A technology for synthesizing magnesium silicate and mechanochemistry, applied in chemical instruments and methods, inorganic chemistry, silicon compounds, etc., can solve problems such as difficulties in large-scale production of lithium magnesium silicate, high input and operating costs, and increased viscosity of materials , to achieve the effects of low cost, shortened reaction time, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Precursor preparation:
[0039] (1) 860g water glass (SiO 2 28wt%,Na 2 (09wt%, modulus 3.22) is dissolved in 4L water, then adds 162g H rapidly while stirring 2 SO 4 ;
[0040] (2) 560g MgCl 2 ·6H 2 O dissolved in 1L of water;
[0041] (3) Add the solution of step (2) into the slurry obtained in step (1), and stir evenly;
[0042] (4) Keep stirring continuously and slowly add 2.4L of 3N NaOH solution to make coprecipitation precursor slurry;
[0043] (5) centrifuging the co-precipitation precursor slurry, and fully washing the centrifuged filter cake with clear water to obtain the washed slurry;
[0044] (6) 14.5g LiOH·H 2 O is dissolved in 200g of water;
[0045] (7) Add the solution of step (6) into the slurry after washing in step (5), and stir evenly;
[0046] (8) Heating while stirring, so that the temperature of the system reaches 70° C. to obtain a precursor.
[0047]The prepared precursor was input into a nano ball mill, the grinding medium was zirco...
Embodiment 2
[0049] Precursor preparation:
[0050] (1) 800g water glass (SiO 2 12.5wt%, Na 2 O3.9wt%, modulus 3.54) was dissolved in 4L water, and then 143g HNO was added quickly while stirring 3 ;
[0051] (2) 530g MgSO 4 Dissolve in 1L of water;
[0052] (3) Add the solution of step (2) into the slurry obtained in step (1), and stir evenly;
[0053] (4) Keep stirring continuously and slowly add 1L of ammonia water to make co-precipitation precursor slurry.
[0054] (5) centrifuging the co-precipitation precursor slurry, and fully washing the centrifuged filter cake with clear water to obtain the washed slurry;
[0055] (6) 29.4g LiOH·H 2 O is dissolved in 400g of water;
[0056] (7) Add the solution of step (6) into the slurry after washing in step (5), and stir evenly;
[0057] (8) Heating while stirring, so that the temperature of the system reaches 30° C. to obtain a precursor.
[0058] The prepared precursor was input into a nano-sand mill, the grinding medium was yttrium-...
Embodiment 3
[0060] Precursor preparation:
[0061] (1) 880g water glass (SiO 2 12.5wt%, Na 2 (03.9wt%, modulus 3.54) is dissolved in 5L water, then adds 120g HCl rapidly while stirring;
[0062] (2) 620g Mg(NO 3 ) 2 Dissolve in 2L water;
[0063] (3) Add the solution of step (2) into the slurry obtained in step (1), and stir evenly;
[0064] (4) Keep stirring continuously and slowly add 2L of ammonia water to make co-precipitation precursor slurry.
[0065] (5) centrifuging the co-precipitation precursor slurry, and fully washing the centrifuged filter cake with clear water to obtain the washed slurry;
[0066] (6) 25g LiOH·H 2 O is dissolved in 400g of water;
[0067] (7) Add the solution of step (6) into the slurry after washing in step (5), and stir evenly;
[0068] (8) Heating while stirring, so that the temperature of the system reaches 50° C. to obtain a precursor.
[0069] The prepared precursor was input into a peeling machine, the grinding medium was zirconium silicate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com