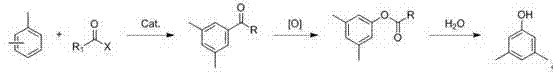
Preparation method of 3,5-dimethylphenol
A technology of dimethylphenol and dimethylphenol ester is applied in the field of preparation of 3,5-dimethylphenol, and achieves the effects of low cost of raw materials, mild reaction conditions and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of 3,5-dimethylacetophenone:
[0025] Add 1.0 mol of acetyl chloride dropwise to the mixture of aluminum trichloride and mixed xylene (2.0 mol of aluminum trichloride and 10.0 mol of xylene) under the condition of ice-water bath, control the dropping temperature not to exceed 15°C, and finish adding Afterwards, the temperature was raised to 100°C and stirred for 5 h. After the reaction, the reaction solution was poured into 1000 ml of ice water. The organic phase was separated, and washed with 0.5% dilute hydrochloric acid, saturated sodium carbonate solution, and water until neutral. After drying with anhydrous sodium sulfate, concentrate under reduced pressure, rectify the concentrate under reduced pressure, and collect the fraction at 94~98℃ / 5mmHg, which is the product 3,5-dimethylacetophenone (yield 89.2%), yellow liquid .
[0026] (2) Synthesis of 3,5-dimethylphenol ethyl ester:
[0027] Add 0.5mol of 3,5-dimethylacetophenone into 1000ml of dichlo...
Embodiment 2
[0031] (1) Synthesis of 3,5-dimethylacetophenone:
[0032]Add 1.0 mol of acetyl chloride dropwise to the mixture of aluminum trichloride and mixed xylene (1.0 mol of aluminum trichloride and 10.0 mol of xylene) in an ice-water bath, and control the dropping temperature not to exceed 15°C. Afterwards, the temperature was raised to 100° C. and stirred for 5 h. After the reaction, the reaction solution was poured into 1000 ml of ice water. The organic phase was separated, and washed with 0.5% dilute hydrochloric acid, saturated sodium carbonate solution, and water until neutral. After drying with anhydrous sodium sulfate, concentrate under reduced pressure, rectify the concentrate under reduced pressure, and collect the fraction at 94~98℃ / 5mmHg, which is the product 3,5-dimethylacetophenone (yield 77.6%), yellow liquid .
[0033] (2) Synthesis of 3,5-dimethylphenol ethyl ester:
[0034] Add 0.5mol of 3,5-dimethylacetophenone into 1000ml of dichloromethane, add 1.0mol of perox...
Embodiment 3
[0038] (1) Synthesis of 3,5-dimethylacetophenone:
[0039] Add 1.0 mol of acetyl chloride dropwise to the mixture of aluminum trichloride and mixed xylene (2.0 mol of aluminum trichloride and 5.0 mol of xylene) under the condition of ice-water bath, control the dropping temperature not to exceed 15°C, and finish adding Afterwards, the temperature was raised to 100° C. and stirred for 5 h. After the reaction, the reaction solution was poured into 1000 ml of ice water. The organic phase was separated, and washed with 0.5% dilute hydrochloric acid, saturated sodium carbonate solution, and water until neutral. After drying with anhydrous sodium sulfate, concentrate under reduced pressure, rectify the concentrate under reduced pressure, and collect the fraction at 94~98℃ / 5 mmHg, which is the product 3,5-dimethylacetophenone (yield 82.3%), yellow liquid.
[0040] (2) Synthesis of 3,5-dimethylphenol ethyl ester:
[0041] Add 0.5mol of 3,5-dimethylacetophenone into 1000ml of ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com