ScFv antibody, encoding gene thereof and application of scFv antibody to preparation of preparation for treating or preventing hepatitis B
A coding and single-chain antibody technology, applied in the field of genetic engineering, can solve the problems of lack of effective preventive measures for emergency prevention of hepatitis B accidents, and achieve the effects of avoiding the spread of diseases, reducing costs, and increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1. Acquisition of anti-preS1scFv single-chain antibody encoding gene
[0073] 1. Initial sample
[0074] The peripheral blood leukocytes of blood donors with high titers of surface antigen antibodies were used as the initial samples as the source of antibody library genes. This can not only increase the kurtosis of natural antibodies, but also select neutralizing antibodies against popular strains, which can achieve multiplier effect with half the effort.
[0075] 2. RNA extraction
[0076] Take fresh peripheral blood leukocytes 10 6 Add 1 ml of cold TRIzol to a pre-cooled Eppendorf (Ep) tube, pipet repeatedly, mix well, place on ice for 10 min, centrifuge at 12000 rpm for 10 min at 4°C. Transfer the supernatant to a new Ep tube, add 200 μl of pre-cooled phenol-chloroform (phenol:chloroform=1:5, volume ratio), shake vigorously for 30 sec, centrifuge at 12000 rpm and 4°C for 10 min. The supernatant was taken, and 200 μl of phenol-chloroform was repeatedly add...
Embodiment 2
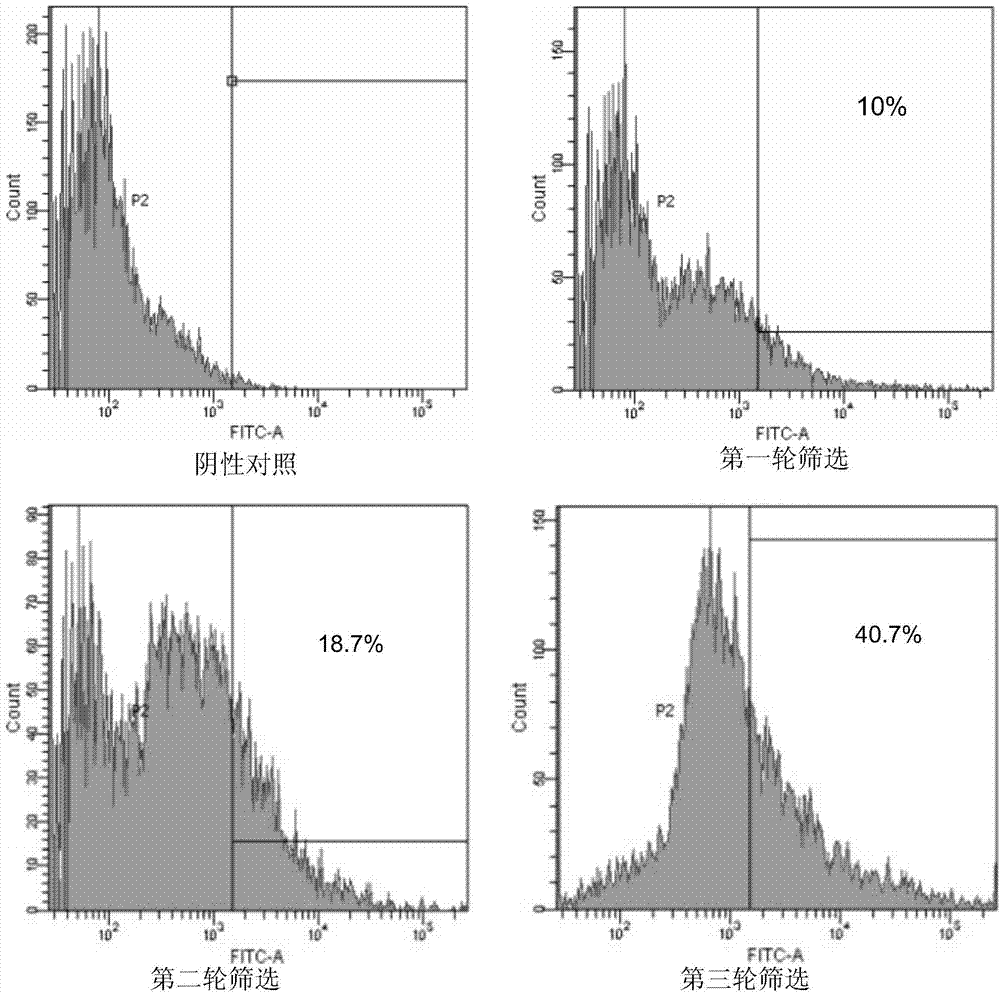
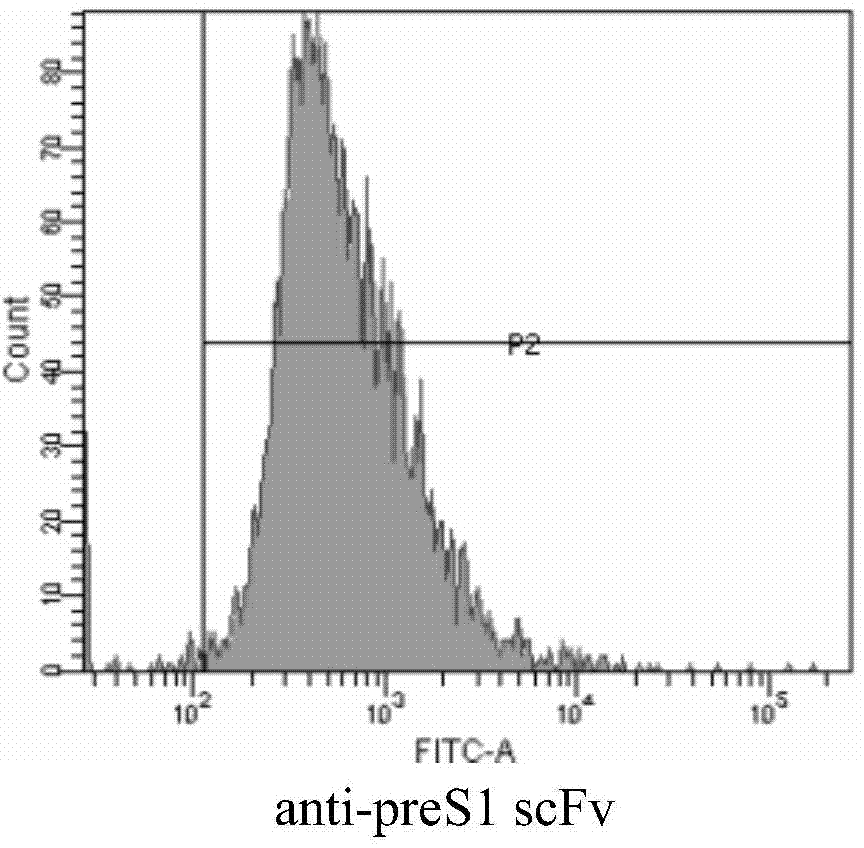
[0195] Example 2. Preparation of anti-preS1scFv single-chain antibody
[0196] 1. Construction of single-chain antibody expression vector (pET-27b-anti-preS1scFv)
[0197] The primer design software Primer Premier 5.0 was used to design PCR primers for the cloning of single chain antibody genes. When designing the upstream primers, Nco I restriction sites were added, and XhoI restriction sites were added downstream, which were synthesized by Invitrogen. The specific primer sequences are as follows:
[0198] anti-preS1scFv-F: CCATGG GT-TCGGTGCAGTTGGTG (the underlined part is the recognition sequence of Nco I, the sequence after - is the 1-15th position of sequence 2);
[0199] anti-preS1scFv-R: CTCGAG TTAACGTTTGATATCC (the underlined part is the recognition sequence of XhoI, the sequence after - is the reverse complement of the 753rd-768th position of sequence 2).
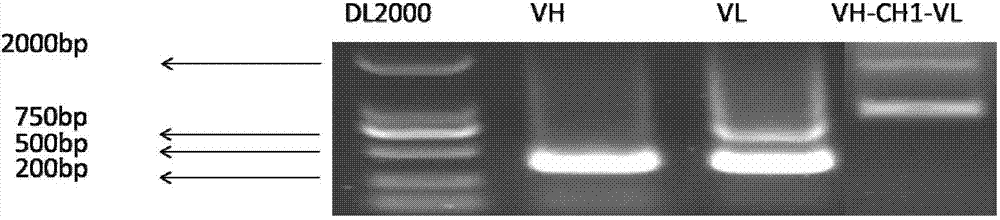
[0200] Using primers, the recombinant plasmid (carrying the DNA fragment shown in SEQ ID NO: 2 in the sequ...
Embodiment 3
[0210] Example 3. Performance detection of anti-preS1scFv single-chain antibody
[0211] 1. ELISA detects the affinity of anti-preS1 scFv antibody
[0212] The anti-preS1 scFv antibody prepared in Example 2 was coated on an ELISA plate overnight at 4°C, with a concentration gradient of 100 μg / mL, 50 μg / mL, 10 μg / mL, and 5 μg / mL, for the detection of anti-preS1 scFv on preS1 The specificity of the protein was also set as a negative control group. After coating overnight, wash 3 times with PBST, 2 min each time, block with 5% (5g / 100ml) nonfat milk powder at 37°C for 2h, after washing, add 10μg / mL preS1 protein (amino acid sequence is sequence 4 in the sequence table) , incubate at 37°C for 1 h, wash as before, add primary antibody (preS1 monoclonal antibody, product of Fitzgerald (Massachusetts, America), whose product catalog number is Cat.No.10-H07A; diluted 2000 times), incubate and wash as above. Then add the secondary antibody (HRP-goat anti-mouse antibody, a product of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com