Alpha-interferon fusion protein preparation and application
A technology of interferon alpha and fusion protein, applied in the field of biomedicine, can solve the problems of dosage change, influence on curative effect, inability to guarantee the stability of interferon alpha fusion protein, etc., and achieve the effect of good stability and reducing particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
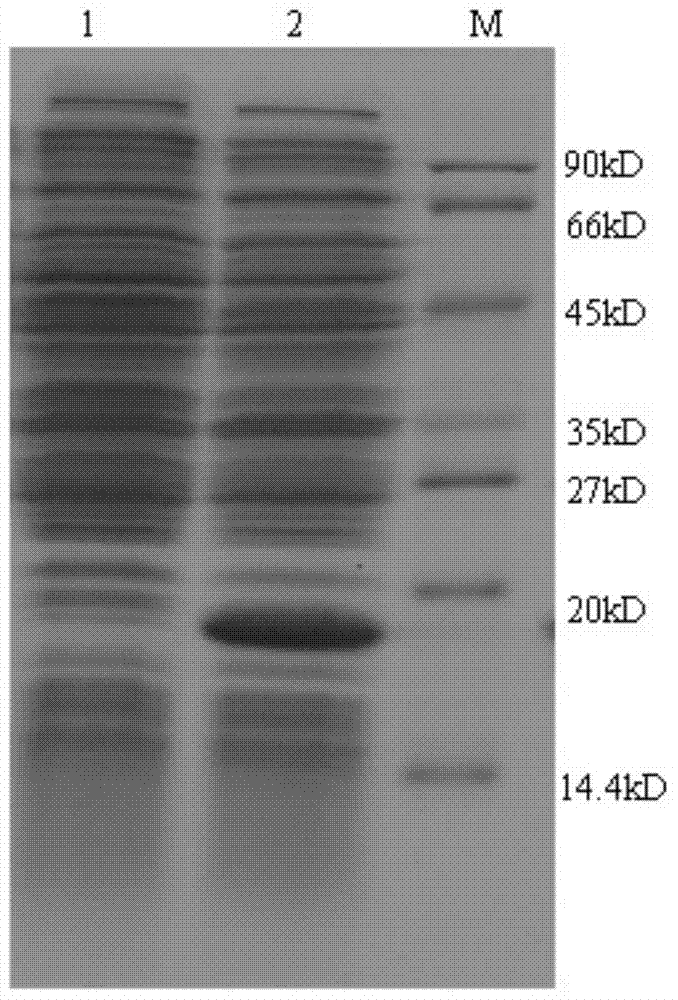
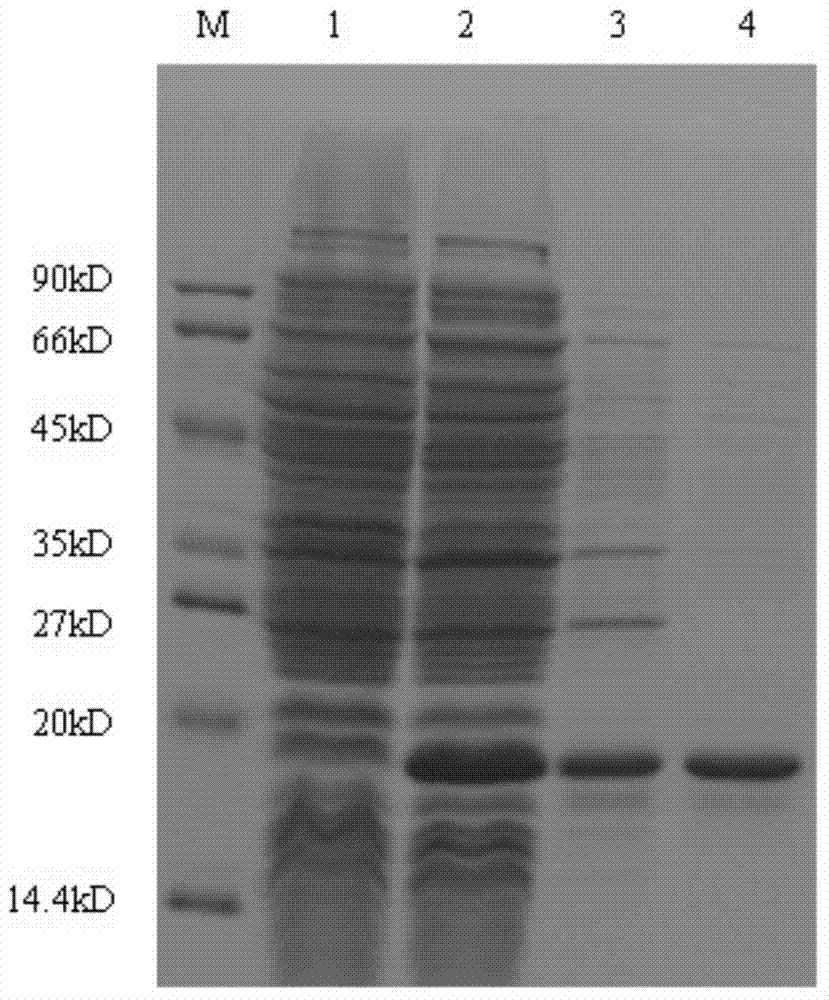
[0027] Embodiment 1 Alpha interferon fusion protein——Preparation, identification and purification of IFNa2-ABP
[0028] A. Screening of human antibody IgG Fc fragment binding peptides. Including the following steps:
[0029] (1) Coating: Dissolve human antibody IgG Fc protein in 0.1M NaHCO at pH 8.6 3 In the solution, make a 100 μg / ml target molecule solution, directly coat it in polystyrene microwells, and incubate overnight at 4°C; pour off the coating solution the next day, remove the residual night, add blocking solution, and act at 4°C for 1 hour ; Remove the blocking solution and wash 6 times with TBST buffer.
[0030] (2) One round of panning: dilute 10 μl phage library with 100 μl TBST buffer, add to the coated microwell, shake gently at room temperature for 1 hour; remove unbound phage, wash 10 times with TBST; add 100 μl 0.2M Glycine -HCl (pH 2.2) buffer was used to separate the bound molecules, and 15 μl of 1M Tris-HCl was added to neutralize to obtain a round of...
Embodiment 2
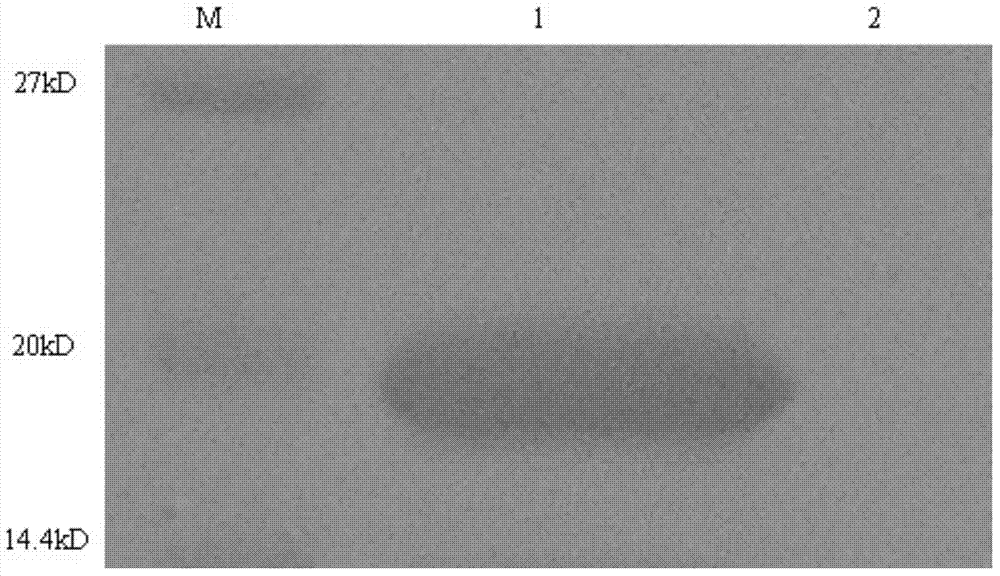
[0065] Embodiment 2Western Blot experiment
[0066] The purpose of this experiment is to confirm that the alpha interferon fusion protein of the present invention can bind to the Fc fragment of human IgG antibody.
[0067] The high-purity IFN2b-ABP purified by hydrophobic chromatography and ion exchange chromatography was subjected to Western Blot experiment. After 15% SDS-PAGE electrophoresis, the protein was transferred to PVDF membrane (200mA, 40min), skimmed milk powder was blocked for 1 hour, human immunoglobulin IgG was added as the primary antibody and incubated overnight at 4°C. After the primary antibody treatment was completed, HRP (horseradish peroxidase)-labeled secondary antibody was added to treat at room temperature for 1.5 hours, and the DAB chromogenic solution was used for color reaction. result( Figure 4 ) shows that there is an obvious color reaction at the IFN2b-ABP fusion protein on the membrane, that is, the protein has a specific binding reaction wit...
Embodiment 3
[0068] Embodiment 3 anti-tumor cell proliferation experiment
[0069] Inoculate DAUDI cells at an appropriate concentration in cell culture medium (180 μL) containing 10% inactivated calf serum, at 37°C, 5% CO 2 , cultivated for one day under saturated humidity, and added purified INF2b-ABP protein and control samples to the cell culture medium the next day (starting from the stock solution with PBS buffer at 10 times, 20 times, 40 times, 80 times, 160 times, 320 times, 640 times, 1280 times, 2560 times dilution), 37°C, 5% CO 2 , cultured at saturated humidity for six days, then added 20 μL of MTT with a concentration of 5 mg / mL, incubated at 37°C for 4 hours, then added triple solution, and kept at 37°C overnight, and measured the A570nm absorbance value with a microplate reader to indicate the level of cell proliferation. The results proved that the INF2b-ABP protein can effectively inhibit the proliferation of tumor cells, as shown in Table 2.
[0070] Table 2
[0071] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com