Sucrose isomerase mutant with improved thermal stability and catalytic efficiency
A technology of sucrose isomerase and mutants, which is applied in the fields of genetic engineering and enzyme engineering, and can solve problems such as reduced catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of site-directed mutants of sucrose isomerase
[0030] (1) Construction of sucrose isomerase site-directed mutants
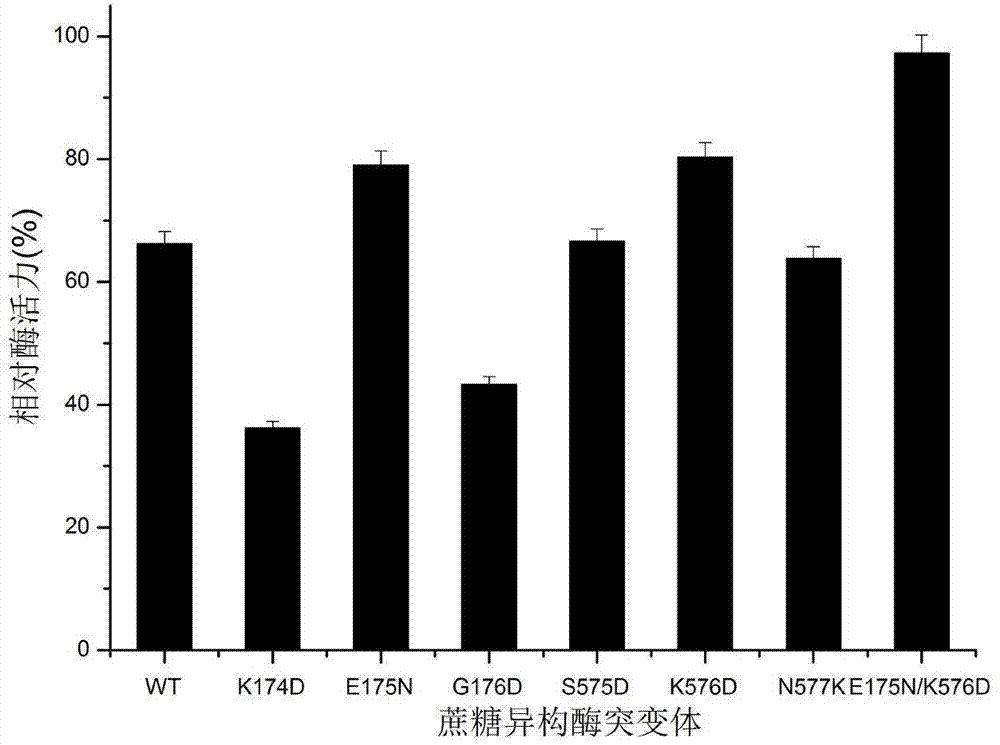
[0031] Two site-directed mutants of sucrose isomerase E175N and E175N / K576D from S. plymuthica:
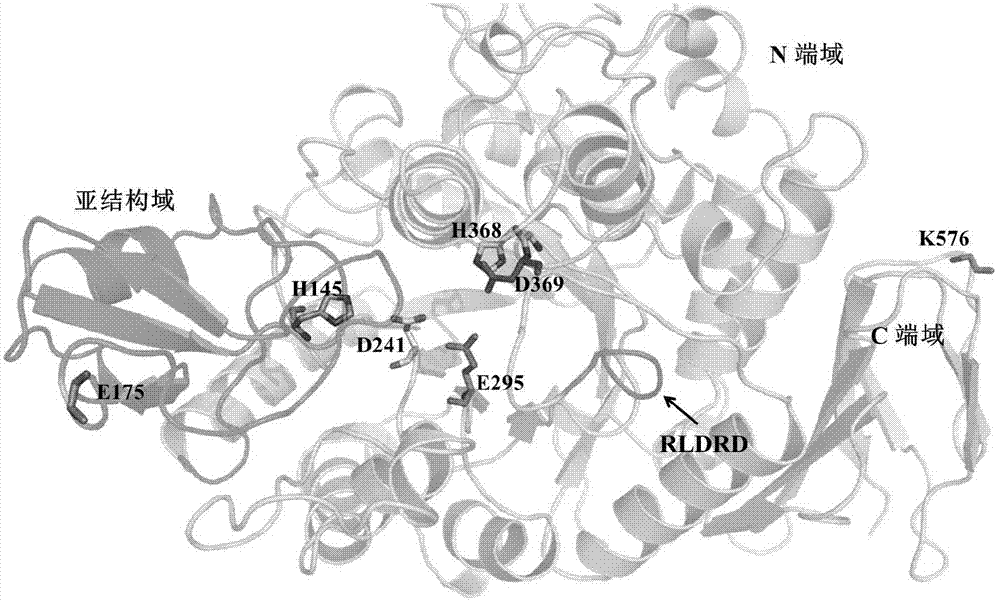
[0032] In the present invention, with the highest similarity Protaminobacter rubrum CBS 574.77 sucrose isomerase (SmuA) crystal structure (PDB ID: 3GBD) as template, S.plymuthica AS9 sucrose isomerase (PalI AS9) was constructed by EMBI-EBL online server ) of the three-dimensional simulation structure ( picture 1). According to the amino acid primary sequence alignment, there is only one amino acid difference between SmuA and PalI AS9, and the similarity reaches 99.86%. Therefore, it can be considered that PalI AS9 has almost the same three-dimensional structure and B factor parameters of SmuA. Based on the above analysis, 6 amino acid residues with higher B factors in PalI AS9 were selected ( Table 1 ), which were then replaced wi...
Embodiment 2
[0063] Embodiment 2: enzyme activity analysis method
[0064] 1) Enzyme activity assay method
[0065] The enzyme activity of sucrose isomerase was determined by 3,5-dinitrosalicylic acid method (DNS method). Sucrose isomerase catalyzes sucrose to generate isomaltulose, and a small amount of trehalulose, glucose and fructose under certain conditions. Isomaltulose is a reducing sugar, sucrose is a non-reducing sugar, and 3,5-dinitrosalicylic acid is reduced to a brown-red amino complex after co-heating with a reducing sugar solution. The depth is proportional to the amount of reducing sugar, so colorimetry can be performed at a wavelength of 540nm to calculate the enzyme activity. Definition of enzyme activity unit: under the above conditions, the amount of enzyme that releases 1 μmol of isomaltulose per minute at the initial stage of the reaction is regarded as an activity unit.
[0066] Enzyme activity assay steps:
[0067] A. Preheating: Take 1.8ml of 10% sucrose solutio...
Embodiment 3
[0070] Example 3: Temperature Optimum and Thermostability of Sucrose Isomerase Mutants
[0071] Using citric acid-disodium hydrogen phosphate (50 mM) at pH 6.0 as buffer solution, the optimum temperature of native sucrose isomerase and mutants was determined in the temperature range from 20 to 50°C. Such as picture 4(a), the optimum temperature of E175N and E175N / K576D is 35°C, which is 5°C higher than that of natural enzymes; E175N and E175N / K576D retain 80.5% and 86.8% of their activities at 40°C, respectively, which are higher than that of natural enzymes. Natural enzymes retain higher activity at 40°C.
[0072] Dilute the purified natural sucrose isomerase and mutants with 50mM pH 6.0 citric acid-disodium hydrogen phosphate buffer to a protein content of 0.25mg / mL and a pH of 6.0, and place them in a constant temperature water bath at 45°C, every 20min Take a sample once, measure its residual enzyme activity, and compare its stability, such as picture 4(b). The half-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com