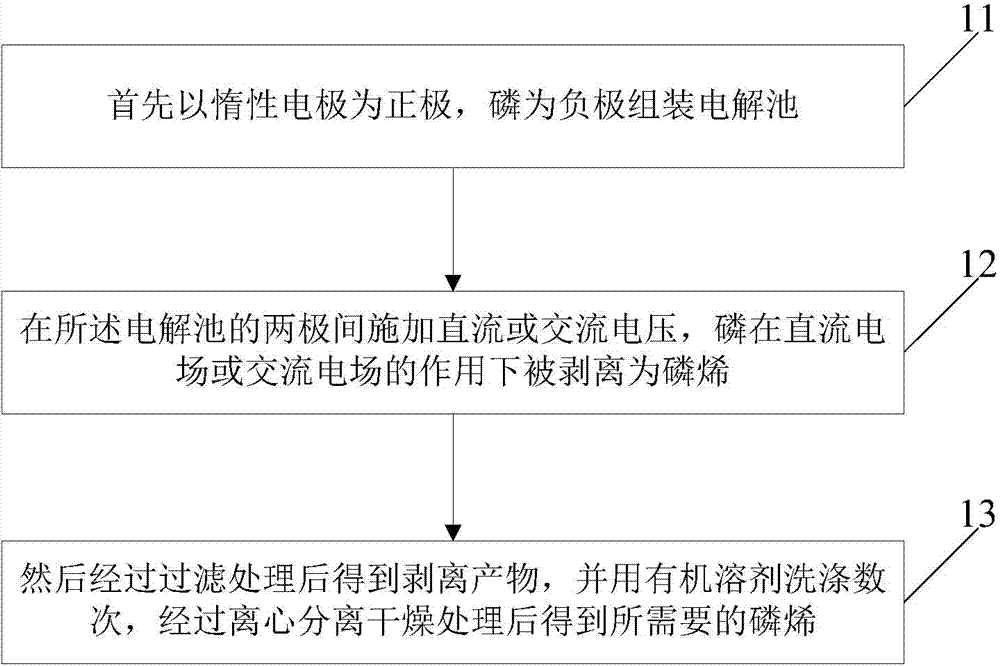
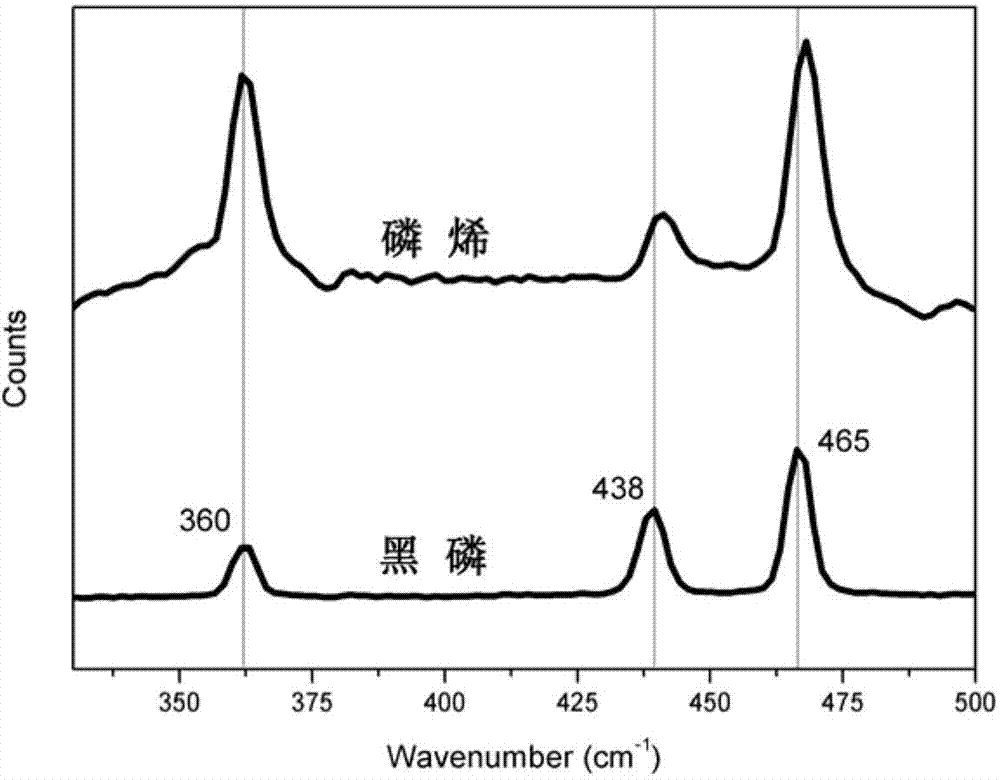
Method for preparing phosphaalkene by utilizing electrochemistry
An electrochemical and phosphorene technology, applied in circuits, electrical components, electrolysis processes, etc., can solve the problems of long time, cumbersome process, low peeling efficiency, etc., and achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Positive electrode: use an area of 1cm 2 , a platinum sheet with a thickness of 0.2mm was used as the positive electrode. Negative electrode: Black phosphorus is directly used as the negative electrode. In a 500ml beaker, NaPF 6 Add it into ethylene carbonate to form a 1mol / L electrolyte solution, and add 0.01g of sodium lauryl sulfate thereto. The above two electrodes were placed in parallel with a distance of 2 cm. Connect the two electrodes with wires as an electrochemical device, and apply a 15V DC voltage between the two electrodes for 20 hours.
[0039]After the exfoliation process was completed, the obtained phosphorene and unexfoliated bulk phosphorus were filtered out, washed several times with ethylene carbonate, and then vacuum-dried at 150 °C to constant weight. Add 100 mg of the obtained phosphorene and unexfoliated massive phosphorus into 20 ml of ethylene carbonate for re-dispersion by ultrasonic vibration for 1 h, the ultrasonic frequency is 15 Hz,...
Embodiment 2
[0042] Positive electrode: A platinum cylinder with a diameter of 3 mm is used as the positive electrode. Negative electrode: Black phosphorus is directly used as the negative electrode. In a 500ml beaker, NaBF 4 Add it to a mixed solution of propylene carbonate and tetrahydrofuran to form a 1mol / L electrolyte, wherein the volume ratio of propylene carbonate and tetrahydrofuran is 1:1. The above two electrodes were placed in parallel with a distance of 1 cm. Connect the two electrodes with wires as an electrochemical device, and apply a 20V DC voltage between the two electrodes for 48 hours.
[0043] After the exfoliation process was completed, the obtained phosphorene and unexfoliated bulk phosphorus were filtered out, washed with propylene carbonate several times, and then vacuum-dried at 150 °C to constant weight. Add 100 mg of the obtained phosphorene and unexfoliated massive phosphorus into 20 ml of propylene carbonate for re-dispersion by ultrasonic vibration for 2 h,...
Embodiment 3
[0045] Positive electrode: use an area of 1cm 2 , a platinum sheet with a thickness of 0.2mm was used as the positive electrode. Negative electrode: black phosphorus and nickel foam are pressed into an area of 1cm by a press 2 electrode sheet and use it as the negative electrode. During the pressing process, the pressure is kept at 15t, and the time is 3min. In a 500ml beaker, the LiPF 6 Add it into a mixed solvent of propylene carbonate and acetonitrile to make a 1.5mol / L electrolyte, wherein the volume ratio of propylene carbonate and acetonitrile is 1:1. The above two electrodes were placed in parallel with a distance of 3 cm. Connect the two electrodes with wires as an electrochemical device, and apply a 2V DC voltage between the two electrodes for 2 hours. Then increase the voltage between positive and negative to 5V. After maintaining the 5V DC voltage for 2 hours, increase the voltage to 20V and continue for 15 minutes, then apply -10V DC voltage to the positi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Sheet thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com