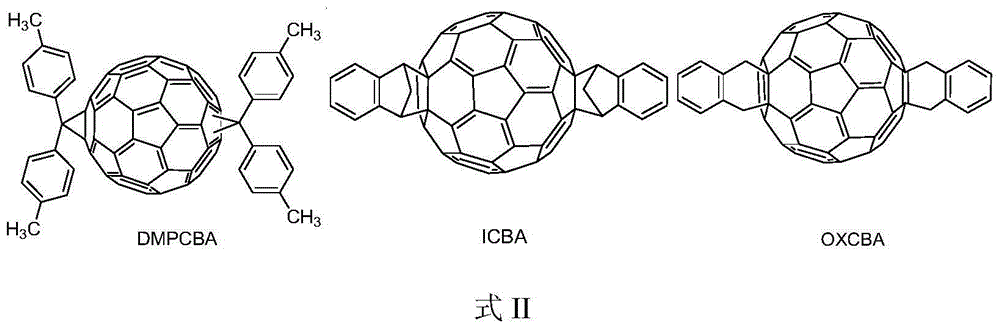
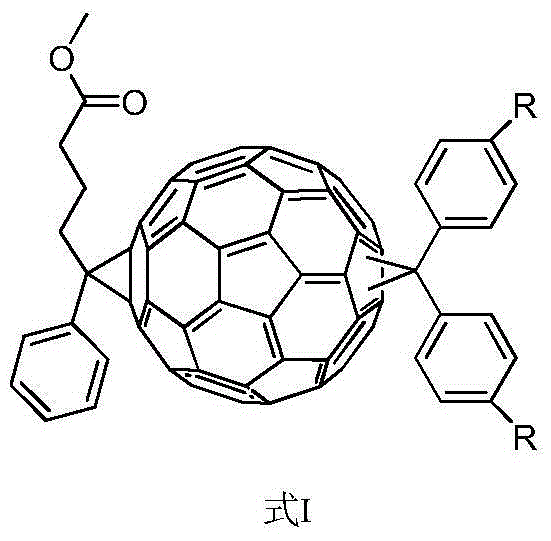
Acceptor material with benzhydryl derivative and PC60BM bis-adduct
A technology of benzhydryl and double adducts is applied in the preparation of organic compounds, organic chemistry, electric solid devices, etc., and can solve the problems of single structure and energy level structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
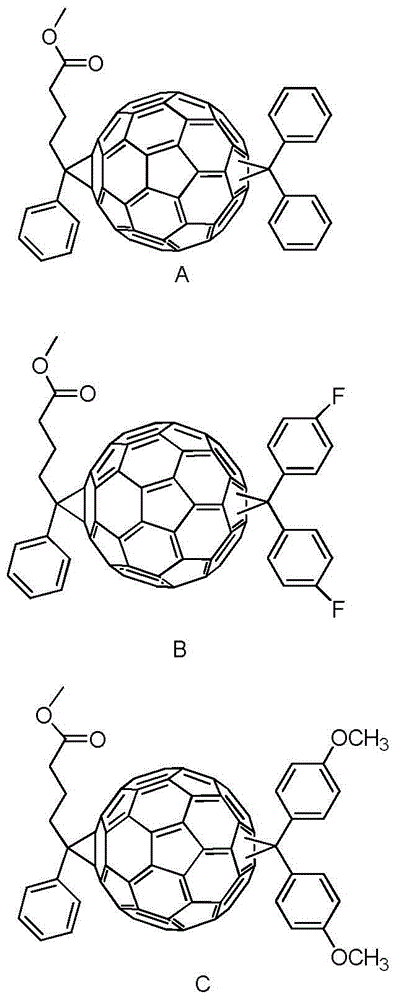
[0021] Example 1: With acceptor benzhydryl PC 60 BM double adduct (DPC 60 BM) synthesis as an example
[0022] The synthetic route is as follows:
[0023]
[0024] Synthesis of benzophenone-tosylhydrazone (compound 1)
[0025] Weigh benzophenone (1 mmol), put p-toluenesulfonyl hydrazide (1.2 equivalents) in a single-necked bottle, and add 10 ml of methanol as solvent. Heat and stir until boiling and reflux. The reaction time was 5.5 hours. After the reaction stopped, let it stand for one day, and then put it into the lower layer of the refrigerator (refrigerator temperature control. -15°C) to cool and crystallize. Part of the solvent should be distilled off first if necessary. White powder or salt crystals were obtained, filtered with a Buchner funnel and washed with a small amount of cold methanol. Put it into a constant temperature vacuum drying oven for drying, control the temperature at 50°C, and vacuum dry overnight. After weighing, it was 3.59g. Yield 96%, pu...
Embodiment 2
[0029] Example 2: With acceptor difluorobenzhydryl PC 60 BM double adduct (DFPC 60 BM) synthesis as an example
[0030] The synthetic route is as follows:
[0031]
[0032] Taking difluorobenzophenone as a raw material, the steps and conditions of Example 1 were used to synthesize compound 3 to obtain target compound 4.
[0033] MALDI-TOF mass spectrometry: DFPC 60 The theoretical value of BM is 1112.0, and the actual value is 1110.11.
Embodiment 3
[0034] Example 3: Using acceptor dimethyl benzhydryl PC 60 BM double adduct (DMPC 60 BM) synthesis as an example
[0035] The synthetic route is as follows:
[0036]
[0037] Using dimethylbenzophenone as a raw material, the target compound 6 was obtained by synthesizing compound 5 using the steps and conditions of Example 1.
[0038] MALDI-TOF mass spectrometry: DMPC 60 The theoretical value of BM is 1104.0, and the actual value is 1104.72.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com